Yonsei Med J.
2020 Apr;61(4):301-309. 10.3349/ymj.2020.61.4.301.
Comparison of In Vivo Pharmacokinetics and Pharmacodynamics of Vancomycin Products Available in Korea
- Affiliations
-
- 1Department of Infectious Diseases, Chonnam National University Medical School, Gwangju, Korea. haroc153@naver.com, sijung@chonnam.ac.kr
- 2Division of Clinical Pharmacology, Department of Pharmacology, Chonnam National University Medical School, Gwangju, Korea.
- 3Division of Infectious Diseases, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Korea.
- KMID: 2471915
- DOI: http://doi.org/10.3349/ymj.2020.61.4.301
Abstract
- PURPOSE
Few studies have been investigated the in vivo efficacy of generic vancomycin products available outside of the United States. In this study, we aimed to compare the in vivo pharmacokinetics (PK) and pharmacodynamics (PD) of five generic vancomycin products available in Korea with those of the innovator.
MATERIALS AND METHODS
The in vitro vancomycin purity of each product was examined using high-pressure liquid chromatography. Single-dose PK analyses were performed using neutropenic mice. The in vivo efficacy of vancomycin products was compared with that of the innovator in dose-effect experiments (25 to 400 mg/kg per day) using a thigh-infection model with neutropenic mice.
RESULTS
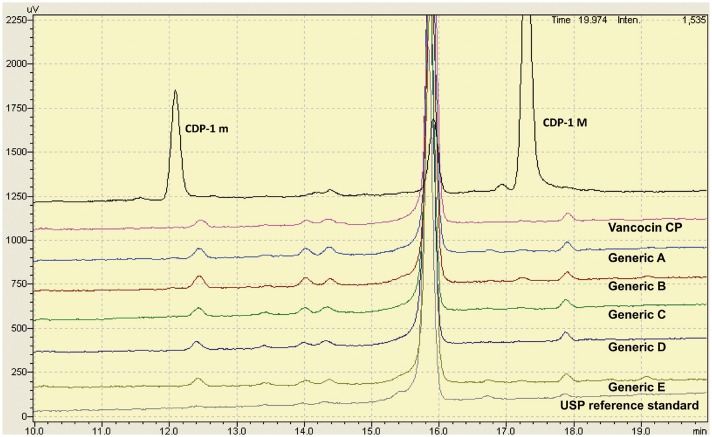
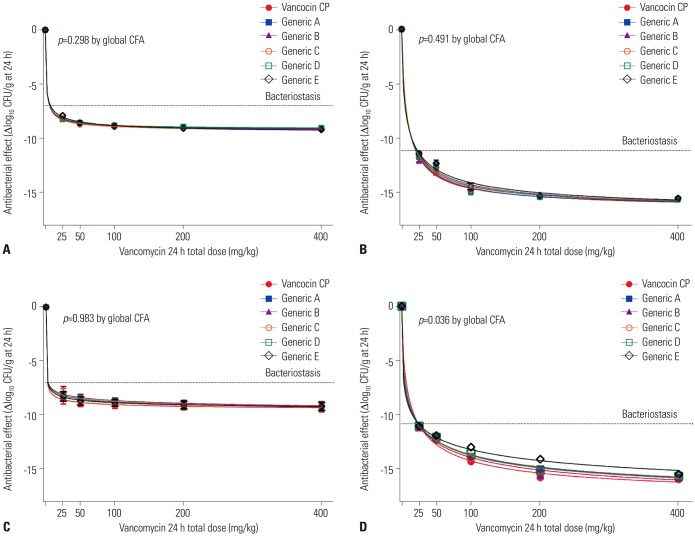
Generic products had a lower proportion of vancomycin B (range: 90.3-93.8%) and a higher proportion of impurities (range: 6.2-9.7%) than the innovator (94.5% and 5.5%, respectively). In an in vivo single-dose PK study, the maximum concentration (C(max)) values of each generic were lower than that of the innovator, and the geographic mean area under the curve ratios of four generics were significantly lower than that of the innovator (all p<0.1). In the thigh-infection model, the maximum efficacies of generic products reflected in maximal effect (E(max)) values were not significantly different from the innovator. However, the PD profile curves of some generic products differed significantly from that of the innovator in mice injected with a high level of Mu3 (all p≤0.05).
CONCLUSION
Some generic vancomycin products available in Korea showed inferior PK and PD profiles, especially in mice infected with hetero-vancomycin-resistant Staphylococcus aureus.
Keyword
MeSH Terms
Figure
Reference
-
1. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998; 339:520–532. PMID: 9709046.2. Jang HC, Choi OJ, Kim GS, Jang MO, Kang SJ, Jung SI, et al. Active surveillance of the trachea or throat for MRSA is more sensitive than nasal surveillance and a better predictor of MRSA infections among patients in intensive care. PLoS One. 2014; 9:e99192. PMID: 24911358.
Article3. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011; 52:e18–e55. PMID: 21208910.
Article4. Jang HC, Kim SH, Kim KH, Kim CJ, Lee S, Song KH, et al. Salvage treatment for persistent methicillin-resistant Staphylococcus aureus bacteremia: efficacy of linezolid with or without carbapenem. Clin Infect Dis. 2009; 49:395–401. PMID: 19569970.5. Jang HC, Kang SJ, Choi SM, Park KH, Shin JH, Choy HE, et al. Difference in agr dysfunction and reduced vancomycin susceptibility between MRSA bacteremia involving SCCmec types IV/IVa and I-III. PLoS One. 2012; 7:e49136. PMID: 23152862.
Article6. Lodise TP Jr, Gotfried M, Barriere S, Drusano GL. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother. 2008; 52:2300–2304. PMID: 18426898.
Article7. van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012; 54:755–771. PMID: 22302374.
Article8. Appelbaum PC. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents. 2007; 30:398–408. PMID: 17888634.
Article9. Henry D, Lexchin J. The pharmaceutical industry as a medicines provider. Lancet. 2002; 360:1590–1595. PMID: 12443614.
Article10. Kirking DM, Ascione FJ, Gaither CA, Welage LS. Economics and structure of the generic pharmaceutical industry. J Am Pharm Assoc (Wash). 2001; 41:578–584. PMID: 11486984.11. Tattevin P, Crémieux AC, Rabaud C, Gauzit R. Efficacy and quality of antibacterial generic products approved for human use: a systematic review. Clin Infect Dis. 2014; 58:458–469. PMID: 24265357.
Article12. Vesga O, Agudelo M, Salazar BE, Rodriguez CA, Zuluaga AF. Generic vancomycin products fail in vivo despite being pharmaceutical equivalents of the innovator. Antimicrob Agents Chemother. 2010; 54:3271–3279. PMID: 20547818.13. Rodriguez CA, Agudelo M, Zuluaga AF, Vesga O. Generic vancomycin enriches resistant subpopulations of Staphylococcus aureus after exposure in a neutropenic mouse thigh infection model. Antimicrob Agents Chemother. 2012; 56:243–247. PMID: 22064531.
Article14. Agudelo M, Rodriguez CA, Gonzalez JM, Zuluaga AF, Vesga O. Some generic of vancomycin prescribed in the United States fail in vivo despite pharmaceutical equivalence (PE) and bioequivalence (BE) demonstrated by the FDA. [abstract A-1323]. In : Poster session presented at the 54th Interscience Conference on Antimi crobial Agents and Chemotherapy (Washington, D.C.); 2014 Sep 5–9; Washington DC.15. Nambiar S, Madurawe RD, Zuk SM, Khan SR, Ellison CD, Faustino PJ, et al. Product quality of parenteral vancomycin products in the United States. Antimicrob Agents Chemother. 2012; 56:2819–2823. PMID: 22314525.
Article16. Hadwiger ME, Sommers CD, Mans DJ, Patel V, Boyne MT 2nd. Quality assessment of U.S. marketplace vancomycin for injection products using high-resolution liquid chromatography-mass spectrometry and potency assays. Antimicrob Agents Chemother. 2012; 56:2824–2830. PMID: 22371900.
Article17. Louie A, Boyne MT 2nd, Patel V, Huntley C, Liu W, Fikes S, et al. Pharmacodynamic evaluation of the activities of six parenteral vancomycin products available in the United States. Antimicrob Agents Chemother. 2015; 59:622–632. PMID: 25385113.
Article18. Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 9th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2012. CLSI document M07-A9.19. Lee DG, Murakami Y, Andes DR, Craig WA. Inoculum effects of ceftobiprole, daptomycin, linezolid, and vancomycin with Staphylococcus aureus and Streptococcus pneumoniae at inocula of 10(5) and 10(7) CFU injected into opposite thighs of neutropenic mice. Antimicrob Agents Chemother. 2013; 57:1434–1441. PMID: 23295932.20. Shin HH, Han S, Yim DS, Lee DG, Park C, Kim SH, et al. Efficacy of vancomycin against Staphylococcus aureus according to inoculum size in a neutropenic mouse infection model. Infect Chemother. 2011; 43:251–257.21. Pachón-Ibáñez ME, Ribes S, Domínguez MA, Fernández R, Tubau F, Ariza J, et al. Efficacy of fosfomycin and its combination with linezolid, vancomycin and imipenem in an experimental peritonitis model caused by a Staphylococcus aureus strain with reduced susceptibility to vancomycin. Eur J Clin Microbiol Infect Dis. 2011; 30:89–95. PMID: 20844913.
Article22. Peetermans WE, Hoogeterp JJ, Hazekamp-van Dokkum AM, van den Broek P, Mattie H. Antistaphylococcal activities of teicoplanin and vancomycin in vitro and in an experimental infection. Antimicrob Agents Chemother. 1990; 34:1869–1874. PMID: 2149812.
Article23. Nagano R, Shibata K, Naito T, Fuse A, Asano K, Hashizume T, et al. Therapeutic efficacy of BO-3482, a novel dithiocarbamate carbapenem, in mice infected with methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1997; 41:2278–2281. PMID: 9333062.
Article24. Reyes N, Skinner R, Benton BM, Krause KM, Shelton J, Obedencio GP, et al. Efficacy of telavancin in a murine model of bacteraemia induced by methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2006; 58:462–465. PMID: 16735425.
Article25. US Food and Drug Administration. Center for Drug Evaluation and Research. Guidance for industry: bioavailability and bioequivalence studies submitted in NDAs or INDs—General considerations. Rockville, MD: Food and Drug Administration;2014.26. European Medicines Agency. Committee for Medicinal Products for Human Use. Guideline on the investigation of bioequivalence. London: European Medicines Agency;2010.27. Agudelo M, Vesga O. Therapeutic equivalence requires pharmaceutical, pharmacokinetic, and pharmacodynamic identities: true bioequivalence of a generic product of intravenous metronidazole. Antimicrob Agents Chemother. 2012; 56:2659–2665. PMID: 22330928.
Article28. Rodriguez CA, Agudelo M, Gonzalez JM, Vesga O, Zuluaga AF. An optimized mouse thigh infection model for enterococci and its impact on antimicrobial pharmacodynamics. Antimicrob Agents Chemother. 2015; 59:233–238. PMID: 25348523.
Article29. Rodriguez CA, Agudelo M, Zuluaga AF, Vesga O. In vivo pharmacodynamics of piperacillin/tazobactam: implications for antimicrobial efficacy and resistance suppression with innovator and generic products. Int J Antimicrob Agents. 2017; 49:189–197. PMID: 27988068.
Article30. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016; 7:27–31. PMID: 27057123.
Article31. Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2013; 57:1654–1663. PMID: 23335735.
Article32. Tattevin P, Saleh-Mghir A, Davido B, Ghout I, Massias L, Garcia de la Maria C, et al. Comparison of six generic vancomycin products for treatment of methicillin-resistant Staphylococcus aureus experimental endocarditis in rabbits. Antimicrob Agents Chemother. 2013; 57:1157–1162. PMID: 23254435.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacokinetics and pharmacodynamics of drugs for sedation
- Therapeutic Drug Monitoring (TDM) of Antimicrobial Agents
- Pharmacokinetics and Pharmacodynamics of Antibiotics : General Concepts and Recent Advances
- Current Updates in Pharmacokinetics and Pharmacodynamics of Fluoroquinolones
- How to design intravenous anesthetic dose regimens based on pharmacokinetics and pharmacodynamics principles