Infect Chemother.
2008 Jun;40(3):133-139. 10.3947/ic.2008.40.3.133.
Therapeutic Drug Monitoring (TDM) of Antimicrobial Agents
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Kyungpook National University College of Medicine, Daegu, Korea. ksw2kms@knu.ac.kr
- KMID: 1782285
- DOI: http://doi.org/10.3947/ic.2008.40.3.133
Abstract
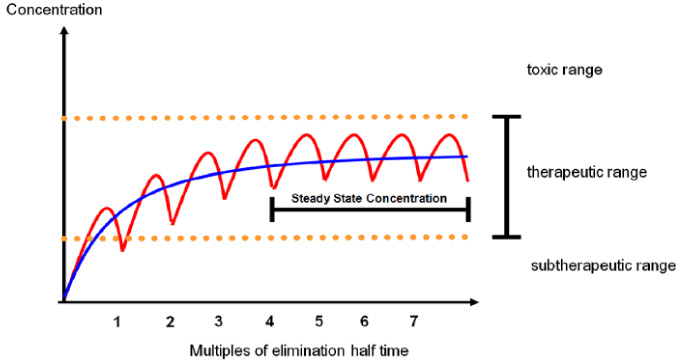
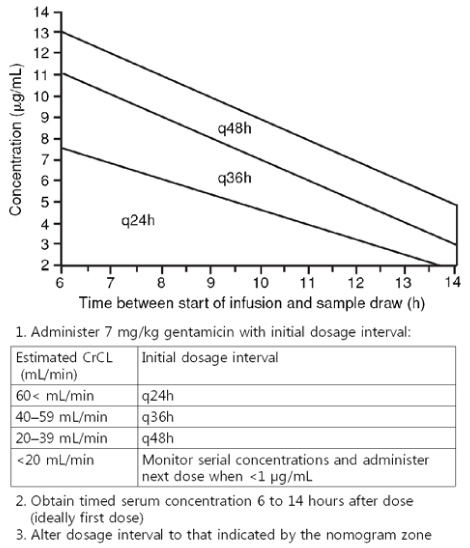
- Optimal exposure of certain antimicrobials and minimization of their toxicity can be achieved by therapeutic drug monitoring (TDM) of antimicrobial agents. Vancomycin and aminoglycosides are most frequently monitored because of narrow therapeutic index and potential adverse effects. The rationale of TDM based on the individual variability in pharmacokinetics (PK) and established correlation between the concentration of the drug and its therapeutic or toxic effects. Benefits of TDM are well established with many studies, the usefulness of antimicrobial TDM for the patients with risk factors of adverse effects (nephrotoxicity and ototoxicity of aminoglycoside and vancomycin) and with treatment failure or severe infection have to be emphasized. With the recent knowledge of PK and pharmacodynamics (PD), interpretation of TDM could be performed with individual PK/PD in regarding to minimal inhibitory concentration (MIC) of specific identified organism or MIC90 of suspected pathogen.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Principles of selecting appropriate antimicrobial agents
Su-Mi Choi, Dong-Gun Lee
J Korean Med Assoc. 2019;62(6):335-344. doi: 10.5124/jkma.2019.62.6.335.Optimal Use and Need for Therapeutic Drug Monitoring of Teicoplanin in Children: A Systematic Review
Joon-sik Choi, Seo Hee Yoon, Hyo Jung Park, Soo-Youn Lee, Yae-Jean Kim
J Korean Med Sci. 2023;38(7):e62. doi: 10.3346/jkms.2023.38.e62.
Reference
-
1. Valles J, Rello J, Ochagavia A, Garnacho J, Alcala MA. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest. 2003. 123:1615–1624.
Article2. Rello J, Rodriguez A. Improving survival for sepsis: on the cutting edge. Crit Care Med. 2003. 31:2807–2808.3. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother. 2005. 49:1306–1311.
Article4. Kuti EL, Patel AA, Coleman CI. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J Crit Care. 2008. 23:91–100.
Article5. Dupont H, Mentec H, Sollet JP, Bleichner G. Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med. 2001. 27:355–362.
Article6. Cosgrove SE, Kaye KS, Eliopoulous GM, Carmeli Y. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch Intern Med. 2002. 162:185–190.
Article7. Benet LZ, Mancinelli L. Swarbrick J, Boylan J C., editors. Clinical Pharmacokinetics and Pharmacodynamics. Encyclopedia of Pharmaceutical Technology. 2006. 1st ed. 572–590.8. Schentag J, Meagher A, RW J. Burton ME, Shaw L, Schentag JJ, Evans E, editors. Applied Pharmacokinetics & Pharmacodynamics: Principle of Therapeutic Drug Monitoring. Aminoglycosides. 2006. 3rd ed. Lippincott Williams and Wilkins;285–327.9. Dasgupta A, Hammett-Stabler C, Broussard L. Hammett-Stabler C, Dasgupta A, editors. Therapeutic Drug Monitoring Data, A Concise Guide. Therapeutic Drug Monitoring of Antimicrobial and Antiviral Agents. 2007. 3rd ed. Washington DC: AACP Press.10. Gilbert D, Moellering R, Eliopoulos G, Sande M, editors. The Sanford Guide to Antimicrobial Therapy 2008. 2008. 38st ed. Sperryville, VA: 93.11. Gilbert D, Moellering R, Eliopoulos G, Sande M, editors. The Sanford Guide to Antimicrobial Therapy 2008. 2008. 38st ed. Sperryville: VA;88.12. Sato M, Chida K, Suda T, Muramatsu H, Suzuki Y, Hashimoto H, Gemma H, Nakamura H. Recommended initial loading dose of teicoplanin, established by therapeutic drug monitoring, and outcome in terms of optimal trough level. J Infect Chemother. 2006. 12:185–189.
Article13. Wertheimer BZ, Freedberg KA, Walensky RP, Yazdanapah Y, Losina E. Therapeutic drug monitoring in HIV treatment: a literature review. HIV Clin Trials. 2006. 7:59–69.
Article14. Smith J, Andes D. Therapeutic drug monitoring of antifungals: pharmacokinetic and pharmacodynamic considerations. Ther Drug Monit. 2008. 30:167–172.
Article15. Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin Infect Dis. 2007. 44:79–86.
Article16. Moore RD, Smith CR, Lietman PS. The association of aminoglycoside plasma levels with mortality in patients with gram-negative bacteremia. J Infect Dis. 1984. 149:443–448.
Article17. Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987. 155:93–99.
Article18. Moise PA, Forrest A, Bhavnani SM, Birmingham MC, Schentag JJ. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am J Health Syst Pharm. 2000. 57:Suppl 2. S4–S9.
Article19. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004. 43:925–942.
Article20. Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006. 42:Suppl 1. S35–S39.
Article21. Begg EJ, Barclay ML, Kirkpatrick CM. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol. 2001. 52:Suppl 1. 35S–43S.
Article22. Kim JH, Kim BK, Lee SY, Chun S, Kwon GC, Yoon Y, Lim JB, Shin DH, Song KE, Lee KR, Suh SP, Kim JQ. Annual Report on External Quality Assessment in Therapeutic Drug Monitoring in Korea (2005). J Lab Med Qual Assur. 2006. 28:121–134.23. Kim JH, Kim BK, Lee SY, Chun S, Kwon GC, Yoon Y, Lim JB, Shin DH, Song KE, Lee KR, Suh SP, Kim JQ. Annual Report on External Quality Assessment in Therapeutic Drug Monitoring in Korea (2004). J Lab Med Qual Assur. 2005. 27:111–124.24. Wie SH, Kim SI, Kim YR, Bae SM, Hong KJ, Ra HO, Kang MW. Therapeutic Drug Monitoring of Vancomycin. Korean J Infect Dis. 2000. 32:141–147.25. Song YG, Kim HK, Roe EK, Lee SY, Ahn BS, Kim JH, Park MS, Yoon HJ, Kim JM. Therapeutic Drug Monitoring of Vancomycin in Korean Patients. Infect Chemother. 2004. 36:311–318.26. Christensen S, Ladefoged K, Frimodt-Møller N. Experience with once daily dosing of gentamicin: considerations regarding dosing and monitoring. Chemotherapy. 1997. 43:442–450.
Article27. Logsdon BA, Phelps SJ. Routine monitoring of gentamicin serum concentrations in pediatric patients with normal renal function is unnecessary. Ann Pharmacother. 1997. 31:1514–1518.
Article28. Duffull SB, Begg EJ. Vancomycin toxicity. What is the evidence for dose dependency? Adverse Drug React Toxicol Rev. 1994. 13:103–114.29. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984. 25:433–437.
Article30. Tobin CM, Darville JM, Thomson AH, Sweeney G, Wilson JF, MacGowan AP, White LO. Vancomycin therapeutic drug monitoring: is there a consensus view? The results of a UK National External Quality Assessment Scheme (UK NEQAS) for Antibiotic Assays questionnaire. J Antimicrob Chemother. 2002. 50:713–718.
Article31. Lee HJ, Kim YM, Choi HO, Sohn JW, Kim MJ. The Usefulness of Therapeutic Drug Monitoring of Vancomycin. J Kor Soc Health-Syst Pharm. 2006. 23:323–329.32. Freeman CD, Quintiliani R, Nightingale CH. Vancomycin therapeutic drug monitoring: is it necessary? Ann Pharmacother. 1993. 27:594–598.
Article33. Saunders NJ. Why monitor peak vancomycin concentrations? Lancet. 1994. 344:1748–1750.
Article34. Edwards DJ, Pancorbo S. Routine monitoring of serum vancomycin concentrations: waiting for proof of its value. Clin Pharm. 1987. 6:652–654.35. Blouin RA, Bauer LA, Miller DD, Record KE, Griffen WO Jr. : Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982. 21:575–580.
Article36. Turnidge JD. The pharmacodynamics of beta-lactams. Clin Infect Dis. 1998. 27:10–22.37. Turnidge J. Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am. 2003. 17:503–528.
Article38. Brunton LL, Lazo JS, Parker KL. Goodman & Gilman's Pharmacology. 2006. McGraw Hill's.39. Begg EJ, Barclay ML. Aminoglycosides-50 years on. Br J Clin Pharmacol. 1995. 39:597–603.40. Lacy MK, Nicolau DP, Nightingale CH, Quintiliani R. The pharmacodynamics of aminoglycosides. Clin Infect Dis. 1998. 27:23–27.
Article41. Smyth AR, Tan KH. Once-daily versus multiple-daily dosing with intravenous aminoglycosides for cystic fibrosis. Cochrane Database Syst Rev. 2006. 3:CD002009.
Article42. Buijk SE, Mouton JW, Gyssens IC, Verbrugh HA, Bruining HA. Experience with a once-daily dosing program of aminoglycosides in critically ill patients. Intensive Care Med. 2002. 28:936–942.
Article43. Nicolau DP, Wu AH, Finocchiaro S, Udeh E, Chow MS, Quintiliani R, Nightingale CH. Once-daily aminoglycoside dosing: impact on requests and costs for therapeutic drug monitoring. Ther Drug Monit. 1996. 18:263–266.
Article44. Begg EJ, Barclay ML, Duffull SB. A suggested approach to once-daily aminoglycoside dosing. Br J Clin Pharmacol. 1995. 39:605–609.
Article45. Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother. 1995. 39:650–655.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Drug Monitoring(TDM) of Psychotropic Drugs
- Pharmacokinetics and Therapeutic Drug Monitoring of Newer Antiepileptic Drugs
- Overview of Therapeutic Drug Monitoring
- Comparison of Trough-Based and Area Under the Curve-Based Therapeutic Drug Monitoring of Vancomycin: An In Silico Study
- Evaluation of the Appropriateness of Antiepileptic Drug Level Monitoring