Endocrinol Metab.
2020 Mar;35(1):64-70. 10.3803/EnM.2020.35.1.64.
Rare PTH Gene Mutations Causing Parathyroid Disorders: A Review
- Affiliations
-
- 1Laboratory of Genomics and Translational Medicine, Gachon University College of Medicine, Incheon, Korea. shleemd@gachon.ac.kr
- 2Department of Surgery, Gachon University College of Medicine, Incheon, Korea.
- 3Department of Internal Medicine, Gachon University College of Medicine, Incheon, Korea.
- KMID: 2471721
- DOI: http://doi.org/10.3803/EnM.2020.35.1.64
Abstract
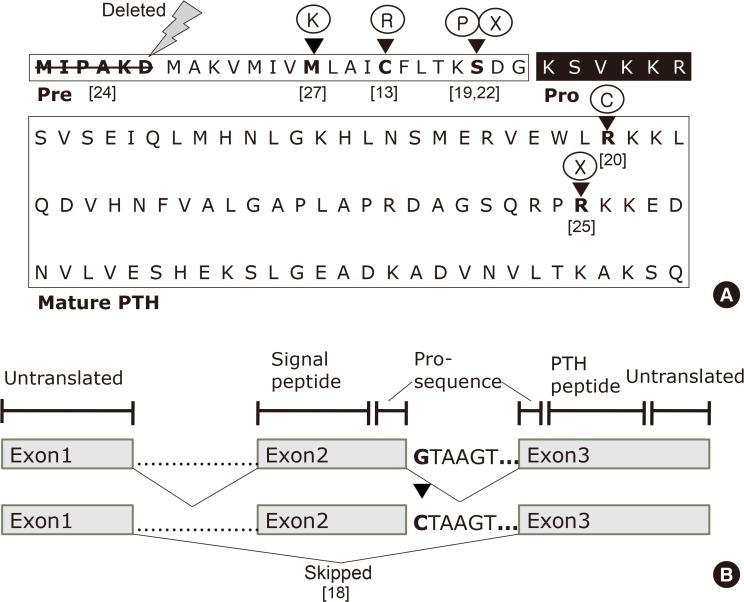
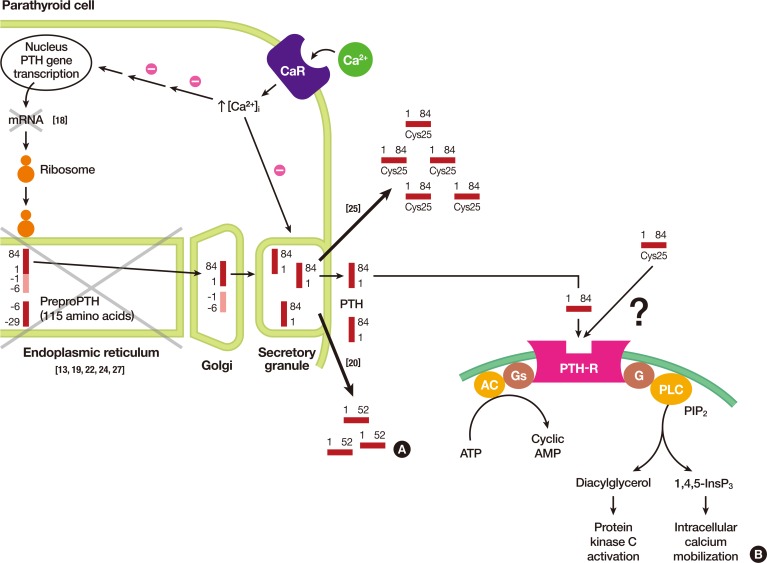
- Since parathyroid hormone (PTH) was first isolated and its gene (PTH) was sequenced, only eight PTH mutations have been discovered. The C18R mutation in PTH, discovered in 1990, was the first to be reported. This autosomal dominant mutation induces endoplasmic reticulum stress and subsequent apoptosis in parathyroid cells. The next mutation, which was reported in 1992, is associated with exon skipping. The substitution of G with C in the first nucleotide of the second intron results in the exclusion of the second exon; since this exon includes the initiation codon, translation initiation is prevented. An S23P mutation and an S23X mutation at the same residue were reported in 1999 and 2012, respectively. Both mutations resulted in hypoparathyroidism. In 2008, a somatic R83X mutation was detected in a parathyroid adenoma tissue sample collected from a patient with hyperparathyroidism. In 2013, a heterozygous p.Met1_Asp6del mutation was incidentally discovered in a case-control study. Two years later, the R56C mutation was reported; this is the only reported hypoparathyroidism-causing mutation in the mature bioactive part of PTH. In 2017, another heterozygous mutation, M14K, was detected. The discovery of these eight mutations in the PTH gene has provided insights into its function and broadened our understanding of the molecular mechanisms underlying mutation progression. Further attempts to detect other such mutations will help elucidate the functions of PTH in a more sophisticated manner.
Keyword
MeSH Terms
Figure
Reference
-
1. Sandstrom I. Omen ny Kortel hos mennisken och atskilige baggdjur. Upsala: Lakarefore rings;1880.2. Gley E. Sur les fonctions du corps thyroïde. C R Soc Biol (Paris). 1891; 43:841–847.3. Erdheim J. Ueber tetania parathyreopriva. Wien Klin Wochenschr. 1906; 19:716–717.4. Collip JB. The extraction of a parathyroid hormone which will prevent or control parathyroid tetany and which regulates the level of blood calcium. J Biol Chem. 1925; 63:395–438.
Article5. Aurbach GD. Isolation of parathyroid hormone after extraction with phenol. J Biol Chem. 1959; 234:3179–3181. PMID: 13848891.
Article6. Rasmussen H, Craig L. Purification of parathyroid hormone by use of counter-current distribution. J Am Chem Soc. 1959; 81:5003.
Article7. Keutmann HT, Sauer MM, Hendy GN, O'Riordan LH, Potts JT Jr. Complete amino acid sequence of human parathyroid hormone. Biochemistry. 1978; 17:5723–5729. PMID: 728431.
Article8. Antonarakis SE, Phillips JA 3rd, Mallonee RL, Kazazian HH Jr, Fearon ER, Waber PG, et al. Beta-globin locus is linked to the parathyroid hormone (PTH) locus and lies between the insulin and PTH loci in man. Proc Natl Acad Sci U S A. 1983; 80:6615–6619. PMID: 6314332.
Article9. Bai M, Quinn S, Trivedi S, Kifor O, Pearce SH, Pollak MR, et al. Expression and characterization of inactivating and activating mutations in the human Ca2+o-sensing receptor. J Biol Chem. 1996; 271:19537–19545. PMID: 8702647.10. Ding C, Buckingham B, Levine MA. Familial isolated hypoparathyroidism caused by a mutation in the gene for the transcription factor GCMB. J Clin Invest. 2001; 108:1215–1220. PMID: 11602629.
Article11. Nesbit MA, Hannan FM, Howles SA, Babinsky VN, Head RA, Cranston T, et al. Mutations affecting G-protein subunit α11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013; 368:2476–2486. PMID: 23802516.12. Ahn TG, Antonarakis SE, Kronenberg HM, Igarashi T, Levine MA. Familial isolated hypoparathyroidism: a molecular genetic analysis of 8 families with 23 affected persons. Medicine (Baltimore). 1986; 65:73–81. PMID: 3005800.13. Arnold A, Horst SA, Gardella TJ, Baba H, Levine MA, Kronenberg HM. Mutation of the signal peptide-encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism. J Clin Invest. 1990; 86:1084–1087. PMID: 2212001.
Article14. Karaplis AC, Lim SK, Baba H, Arnold A, Kronenberg HM. Inefficient membrane targeting, translocation, and proteolytic processing by signal peptidase of a mutant preproparathyroid hormone protein. J Biol Chem. 1995; 270:1629–1635. PMID: 7829495.
Article15. Suprasdngsin C, Wattanachal A, Preeysombat C. Parathyroid hormone gene mutation in patient with hypoparathyroidism (Annual Research Abstract 164). 128. Bangkok: Mahidol University;2001.16. Datta R, Waheed A, Shah GN, Sly WS. Signal sequence mutation in autosomal dominant form of hypoparathyroidism induces apoptosis that is corrected by a chemical chaperone. Proc Natl Acad Sci U S A. 2007; 104:19989–19994. PMID: 18056632.
Article17. Hammer GD, McPhee SJ. Chapter 17, Disorders of the parathyroids & calcium & phosphorus metabolism. Pathophysiology of disease: an introduction to clinical medicine. 7th ed. New York: McGraw-Hill Education;2014.18. Parkinson DB, Thakker RV. A donor splice site mutation in the parathyroid hormone gene is associated with autosomal recessive hypoparathyroidism. Nat Genet. 1992; 1:149–152. PMID: 1302009.
Article19. Sunthornthepvarakul T, Churesigaew S, Ngowngarmratana S. A novel mutation of the signal peptide of the preproparathyroid hormone gene associated with autosomal recessive familial isolated hypoparathyroidism. J Clin Endocrinol Metab. 1999; 84:3792–3796. PMID: 10523031.
Article20. Au AY, McDonald K, Gill A, Sywak M, Diamond T, Conigrave AD, et al. PTH mutation with primary hyperparathyroidism and undetectable intact PTH. N Engl J Med. 2008; 359:1184–1186.21. Lim SK, Gardella TJ, Baba H, Nussbaum SR, Kronenberg HM. The carboxy-terminus of parathyroid hormone is essential for hormone processing and secretion. Endocrinology. 1992; 131:2325–2330. PMID: 1425431.
Article22. Ertl DA, Stary S, Streubel B, Raimann A, Haeusler G. A novel homozygous mutation in the parathyroid hormone gene (PTH) in a girl with isolated hypoparathyroidism. Bone. 2012; 51:629–632. PMID: 22722080.
Article23. Goswami R, Mohapatra T, Gupta N, Rani R, Tomar N, Dikshit A, et al. Parathyroid hormone gene polymorphism and sporadic idiopathic hypoparathyroidism. J Clin Endocrinol Metab. 2004; 89:4840–4845. PMID: 15472173.
Article24. Tomar N, Gupta N, Goswami R. Calcium-sensing receptor autoantibodies and idiopathic hypoparathyroidism. J Clin Endocrinol Metab. 2013; 98:3884–3891. PMID: 23873991.
Article25. Lee S, Mannstadt M, Guo J, Kim SM, Yi HS, Khatri A, et al. A homozygous [Cys25]PTH(1-84) mutation that impairs PTH/PTHrP receptor activation defines a novel form of hypoparathyroidism. J Bone Miner Res. 2015; 30:1803–1813. PMID: 25891861.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two novel rare variants in the PTH gene found in patients with hypoparathyroidism
- Hypoparathyroidism in children and adolescents
- Neonatal transient pseudohypoparathyroidism: could it be included among inactivating parathyroid hormone (PTH)/PTH-related protein signalling disorders?
- The Parathyroid Gland: An Overall Review of the Hidden Organ for Radiologists
- Simultaneous elevation of serum parathyroid hormone(PTH) and parathyroid hormone-related protein(PTHrP) in a case of lung cancer with hypercalcemia