Obstet Gynecol Sci.
2019 Mar;62(2):112-119. 10.5468/ogs.2019.62.2.112.
Effects of cisplatin on photosensitizer-mediated photodynamic therapy in breast tumor-bearing nude mice
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Chosun University College of Medicine, Gwangju, Korea. themoon6pence@hanmail.net
- KMID: 2471683
- DOI: http://doi.org/10.5468/ogs.2019.62.2.112
Abstract
OBJECTIVE
This study aimed to evaluate the potential effects of cisplatin on photodynamic therapy (PDT) in breast cancer using a breast tumor-bearing mouse model.
METHODS
In this study, breast tumor (experimental mammary tumour-6 cell)-bearing nude mice were used as experimental animals. Photolon® (photosensitizer, 2.5 mg/kg body weight [BW]) was injected intraperitoneally; after 2 hours, the tumors were irradiated (660 nm, 80 J/cm2) using a diode laser tool. Cisplatin (3 mg/kg BW) was injected intraperitoneally 1 hour before the Photolon® injection.
RESULTS
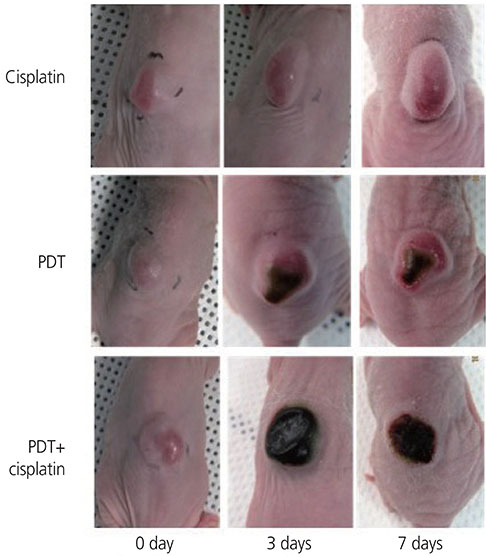
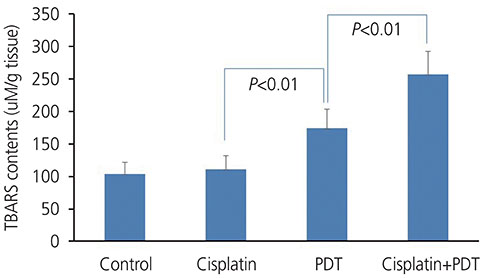
Tumor volume increased over time in the control group and was not different from that in the cisplatin group. In the PDT group, the tumor volume increased on day 3, but not on day 7. In the cisplatin+PDT group, tumor volume increased on day 3 but decreased on day 7. There was no significant difference in the levels of thiobarbituric acid reactive substance (TBARS) in tumor tissues between the control and cisplatin groups. The levels of TBARS in the cisplatin+PDT group were higher (47%) than those in the PDT group. Analysis of tumor tissue transcriptomes showed that the expression of genes related to the inflammatory response including CL and XCL genes increased, while that of Fn1 decreased in the cisplatin+PDT group compared with the PDT group.
CONCLUSION
These results suggest that cisplatin enhances the therapeutic effect of PDT in a breast tumor-bearing mouse model. However, further clinical studies involving patients with breast cancer is needed.
Keyword
MeSH Terms
Figure
Reference
-
1. McGuire A, Brown JA, Malone C, McLaughlin R, Kerin MJ. Effects of age on the detection and management of breast cancer. Cancers (Basel). 2015; 7:908–929.2. Mangla B, Kohli K. Combination of natural agent with synthetic drug for the breast cancer therapy. Int J Drug Dev Res. 2018; 10:22–26.3. Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011; 61:250–281.
Article4. Silva JN, Filipe P, Morlière P, Mazière JC, Freitas JP, Cirne de Castro JL, et al. Photodynamic therapies: principles and present medical applications. Biomed Mater Eng. 2006; 16:S147–54.5. O'Connor AE, Gallagher WM, Byrne AT. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol. 2009; 85:1053–1074.6. Chin WW, Lau WK, Heng PW, Bhuvaneswari R, Olivo M. Fluorescence imaging and phototoxicity effects of new formulation of chlorin e6-polyvinylpyrrolidone. J Photochem Photobiol B. 2006; 84:103–110.
Article7. Petrov P, Trukhacheva T, Isakov G, Gavryilov M, Turyn V, Kravchenko E. Photolon an agent for photodynamic diagnosis and therapy: non-clinical and clinical experience. Acta Bio-Optica et Informatica Medica. 2004; 10:6–7.8. Ge R, Ahn JC, Shin JI, Bahk CW, He P, Chung PS. An in vitro and in vivo study of combination therapy with Photogem®-mediated photodynamic therapy and cisplatin on mouse cancer cells (CT-26). Photomed Laser Surg. 2011; 29:155–160.9. Lim HS. Development and optimization of a diode laser for photodynamic therapy. Laser Ther. 2011; 20:195–203.10. Beyer W. Systems for light application and dosimetry in photodynamic therapy. J Photochem Photobiol B. 1996; 36:153–156.
Article11. Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007; 7:573–584.
Article12. Peddi P, Shi R, Nair B, Ampil F, Mills GM, Jafri SH. Cisplatin, cetuximab, and radiation in locally advanced head and neck squamous cell cancer: a retrospective review. Clin Med Insights Oncol. 2015; 9:1–7.
Article13. Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012; 31:1869–1883.
Article14. Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003; 4:889–901.
Article15. Stathopoulos GP. Cisplatin: process and future. J BUON. 2013; 18:564–569.16. Ali S, Khurshid A, Maqsood M, Rafi M, Khan JA, Zaidi SS, et al. Study of low doses cisplatin synergistic effect on photodynamic outcome of aluminum phythalocyanine on soft tissue sarcoma (RD) cell line. Photodiagn Photodyn Ther. 2015; 12:146–149.
Article17. Wei XQ, Ma HQ, Liu AH, Zhang YZ. Synergistic anticancer activity of 5-aminolevulinic acid photodynamic therapy in combination with low-dose cisplatin on Hela cells. Asian Pac J Cancer Prev. 2013; 14:3023–3028.
Article18. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009; 4:44–57.
Article19. Berg K, Moan J. Lysosomes as photochemical targets. Int J Cancer. 1994; 59:814–822.
Article20. Chen B, Pogue BW, Luna JM, Hardman RL, Hoopes PJ, Hasan T. Tumor vascular permeabilization by vascular-targeting photosensitization: effects, mechanism, and therapeutic implications. Clin Cancer Res. 2006; 12:917–923.
Article21. Johnsson A, Olsson C, Nygren O, Nilsson M, Seiving B, Cavallin-Stahl E. Pharmacokinetics and tissue distribution of cisplatin in nude mice: platinum levels and cisplatin-DNA adducts. Cancer Chemother Pharmacol. 1995; 37:23–31.
Article22. Chekulayeva LV, Shevchuk IN, Chekulayev VA, Ilmarinen K. Hydrogen peroxide, superoxide, and hydroxyl radicals are involved in the phototoxic action of hematoporphyrin derivative against tumor cells. J Environ Pathol Toxicol Oncol. 2006; 25:51–77.
Article23. Brozovic A, Ambriović-Ristov A, Osmak M. The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Crit Rev Toxicol. 2010; 40:347–359.
Article24. Korbelik M. PDT-associated host response and its role in the therapy outcome. Lasers Surg Med. 2006; 38:500–508.
Article25. Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol. 1996; 60:365–371.
Article26. Opdenakker G, Froyen G, Fiten P, Proost P, Van Damme J. Human monocyte chemotactic protein-3 (MCP-3): molecular cloning of the cDNA and comparison with other chemokines. Biochem Biophys Res Commun. 1993; 191:535–542.
Article27. Jia GQ, Gonzalo JA, Lloyd C, Kremer L, Lu L, Martinez-A C, et al. Distinct expression and function of the novel mouse chemokine monocyte chemotactic protein-5 in lung allergic inflammation. J Exp Med. 1996; 184:1939–1951.
Article28. Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989; 86:612–616.
Article29. Persson T, Monsef N, Andersson P, Bjartell A, Malm J, Calafat J, et al. Expression of the neutrophil-activating CXC chemokine ENA-78/CXCL5 by human eosinophils. Clin Exp Allergy. 2003; 33:531–537.
Article30. Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985; 315:672–676.31. Anestakis D, Petanidis S, Kalyvas S, Nday CM, Tsave O, Kioseoglou E, et al. Mechanisms and applications of interleukins in cancer immunotherapy. Int J Mol Sci. 2015; 16:1691–1710.32. Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002; 115:3861–3863.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of quercetin on the anti-tumor activity of cisplatin in EMT6 breast tumor-bearing mice
- In vivo study of photodynamic therapy using a new photosensitizer, 9-hydroxypheophorbide-a and 670 nm diode laser on HT-3 cervical cancer cell
- Photodynamic Therapy for Esophageal Cancer
- Photodynamic Therapy Of Premalignant And Malignant Lesions In Oral And Maxillofacial Surgery
- Photodynamic therapy for breast cancer in a BALB/c mouse model