Obstet Gynecol Sci.
2019 Jul;62(4):242-248. 10.5468/ogs.2019.62.4.242.
Effect of quercetin on the anti-tumor activity of cisplatin in EMT6 breast tumor-bearing mice
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, Chosun University, Gwangju, Korea. ogatg@chosun.ac.kr
- 2Department of Internal Medicine, College of Medicine, Chosun University, Gwangju, Korea.
- KMID: 2451653
- DOI: http://doi.org/10.5468/ogs.2019.62.4.242
Abstract
OBJECTIVE
The purpose of this study was to determine the effect of quercetin on the antitumor activity of cisplatin and its side-effects.
METHODS
EMT6 cells, a mouse breast cancer cell line, were injected subcutaneously in mice to generate a breast tumor-bearing mouse model. Experimental groups were divided into four groups: control (C), quercetin (Q), cisplatin (CP), and cisplatin+quercetin (CP+Q).
RESULTS
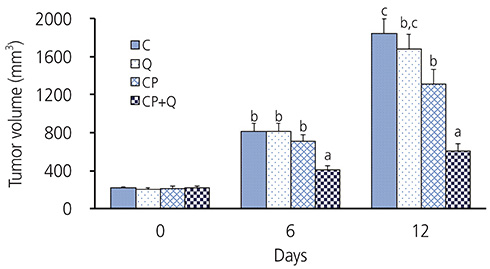
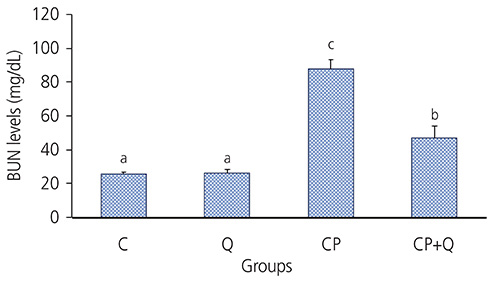
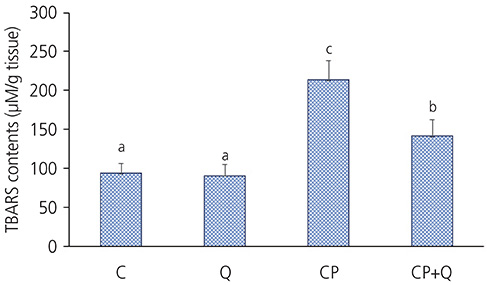
The tumor volume of the CP+Q group was significantly lower than that of the CP group. Serum blood urea nitrogen and creatinine levels in the CP+Q group were lower than those in the CP group. Renal γ-glutamyltranspeptidase and alkaline phosphatase activities were significantly higher in the CP+Q group than in the CP group, and the content of renal thiobarbituric acid reactive substance was significantly lower in the CP+Q group than that in the CP group. These results suggested that quercetin and cisplatin synergistically increased cellular toxicity in breast cancer cells and mediated cancer growth inhibition, thereby enhancing the antitumor effect of cisplatin compared to when only cisplatin was administered. Quercetin also reduced renal toxicity, which arose as a potential a side effect of cisplatin.
CONCLUSION
The enhanced antitumor effect of cisplatin and decreased renal toxicity after quercetin treatment suggested the applicability of quercetin as an adjuvant for chemotherapeutic agents.
Keyword
MeSH Terms
Figure
Reference
-
1. Matsuyama R, Reddy S, Smith TJ. Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol. 2006; 24:3490–3496.
Article2. Lønning PE. Molecular basis for therapy resistance. Mol Oncol. 2010; 4:284–300.
Article3. Chuthapisith S, Eremin JM, El-Sheemy M, Eremin O. Neoadjuvant chemotherapy in women with large and locally advanced breast cancer: chemoresistance and prediction of response to drug therapy. Surgeon. 2006; 4:211–219.
Article4. Vogl DT, Stadtmauer EA. High-dose chemotherapy and autologous hematopoietic stem cell transplantation for metastatic breast cancer: a therapy whose time has passed. Bone Marrow Transplant. 2006; 37:985–987.
Article5. Mishra BB, Tiwari VK. Natural products: an evolving role in future drug discovery. Eur J Med Chem. 2011; 46:4769–4807.
Article6. Hill JM, Loeb E, MacLellan A, Hill NO, Khan A, King JJ. Clinical studies of Platinum Coordination compounds in the treatment of various malignant diseases. Cancer Chemother Rep. 1975; 59:647–659.7. Jordan P, Carmo-Fonseca M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci. 2000; 57:1229–1235.
Article8. Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003; 4:889–901.
Article9. Sharp CN, Siskind LJ. Developing better mouse models to study cisplatin-induced kidney injury. Am J Physiol Renal Physiol. 2017; 313:F835–41.
Article10. Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, et al. The antitumor activities of flavonoids. In Vivo. 2005; 19:895–909.11. Khan F, Niaz K, Maqbool F, Ismail Hassan F, Abdollahi M, Nagulapalli Venkata KC, et al. Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients. 2016; 8:E529.
Article12. Aghapour F, Moghadamnia AA, Nicolini A, Kani SN, Barari L, Morakabati P, et al. Quercetin conjugated with silica nanoparticles inhibits tumor growth in MCF-7 breast cancer cell lines. Biochem Biophys Res Commun. 2018; 500:860–865.
Article13. Behling EB, Sendão MC, Francescato HD, Antunes LM, Costa RS, Bianchi ML. Comparative study of multiple dosage of quercetin against cisplatin-induced nephrotoxicity and oxidative stress in rat kidneys. Pharmacol Rep. 2006; 58:526–532.14. Sánchez-González PD, López-Hernández FJ, Dueñas M, Prieto M, Sánchez-López E, Thomale J, et al. Differential effect of quercetin on cisplatin-induced toxicity in kidney and tumor tissues. Food Chem Toxicol. 2017; 107:226–236.
Article15. Zhang X, Guo Q, Chen J, Chen Z. Quercetin enhances cisplatin sensitivity of human osteosarcoma cells by modulating microRNA-217-KRAS Axis. Mol Cells. 2015; 38:638–642.
Article16. Arzuman L, Beale P, Chan C, Yu JQ, Huq F. Synergism from combinations of tris(benzimidazole) monochloroplatinum(II) chloride with capsaicin, quercetin, curcumin and cisplatin in human ovarian cancer cell lines. Anticancer Res. 2014; 34:5453–5464.17. Tate SS, Meister A. γ-glutamyl transpeptidase from kidney. In : Meister A, editor. Methods in enzymology. Volume 113. New York (NY): Academic Press;1985. p. 400–437.18. Tenenhouse HS, Scriver CR, Vizel EJ. Alkaline phosphatase activity does not mediate phosphate transport in the renal-cortical brush-border membrane. Biochem J. 1980; 190:473–476.
Article19. Li QC, Liang Y, Hu GR, Tian Y. Enhanced therapeutic efficacy and amelioration of cisplatin-induced nephrotoxicity by quercetin in 1,2-dimethyl hydrazine-induced colon cancer in rats. Indian J Pharmacol. 2016; 48:168–171.
Article20. Fatima S, Arivarasu NA, Mahmood R. Vitamin C attenuates cisplatin-induced alterations in renal brush border membrane enzymes and phosphate transport. Hum Exp Toxicol. 2007; 26:419–426.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of cisplatin on photosensitizer-mediated photodynamic therapy in breast tumor-bearing nude mice
- Immunotherapy with methyl gallate, an inhibitor of Treg cell migration, enhances the anti-cancer effect of cisplatin therapy
- Antitumor and Apoptosis Induction Effects of Paeonol on Mice Bearing EMT6 Breast Carcinoma
- Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity
- Anticarcinogenic effect of quercetin by inhibition of insulin-like growth factor (IGF)-1 signaling in mouse skin cancer