Allergy Asthma Immunol Res.
2020 May;12(3):412-429. 10.4168/aair.2020.12.3.412.
Relationship of Microbial Profile With Airway Immune Response in Eosinophilic or Neutrophilic Inflammation of Asthmatics
- Affiliations
-
- 1Department of Interdisciplinary Program in Biomedical Science Major, Graduate School, Soonchunhyang University, Asan, Korea. hschang@sch.ac.kr
- 2Department of Internal Medicine, Korean Armed Forces Capital Hospital, Seongnam, Korea.
- 3Genome Research Center and Division of Allergy and Respiratory Medicine, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea. newstart1221@naver.com
- KMID: 2471226
- DOI: http://doi.org/10.4168/aair.2020.12.3.412
Abstract
- PURPOSE
Different characteristics of airway microbiome in asthmatics may lead to differential immune responses, which in turn cause eosinophilic or neutrophilic airway inflammation. However, the relationships among these factors have yet to be fully elucidated.
METHODS
Microbes in induced sputum samples were subjected to sequence analysis of 16S rRNA. Airway inflammatory phenotypes were defined as neutrophils (>60%) and eosinophils (>3%), and inflammation endotypes were defined by levels of T helper (Th) 1 (interferon-γ), Th2 (interleukin [IL]-5 and IL-13), Th-17 (IL-17), and innate Th2 (IL-25, IL-33, and thymic stromal lymphopoietin) cytokines, inflammasomes (IL-1β), epithelial activation markers (granulocyte-macrophage colony-stimulating factor and IL-8), and Inflammation (IL-6 and tumor necrosis factor-α) cytokines in sputum supernatants was assessed by enzyme-linked immunosorbent assay.
RESULTS
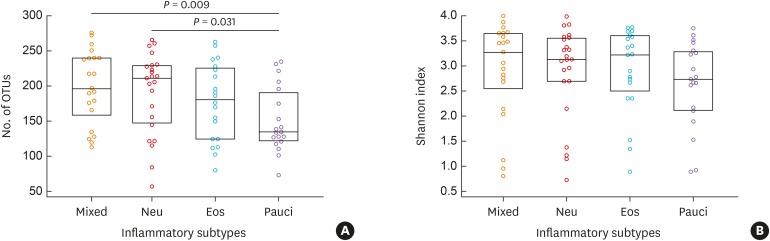
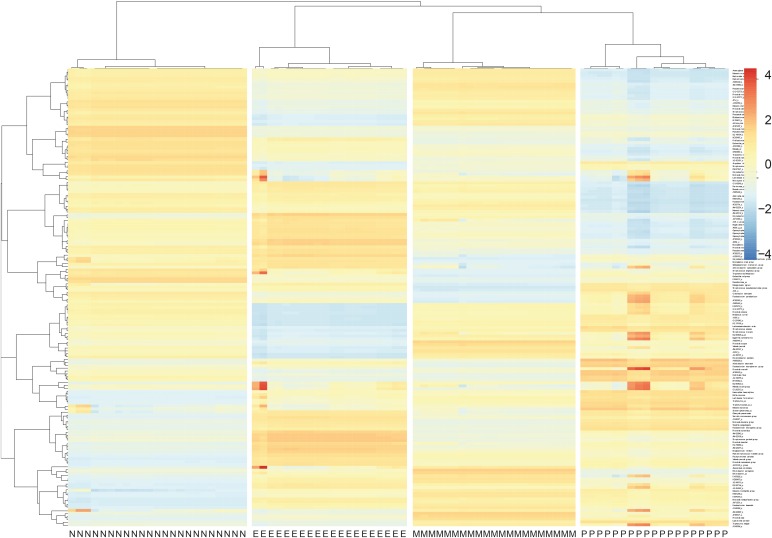
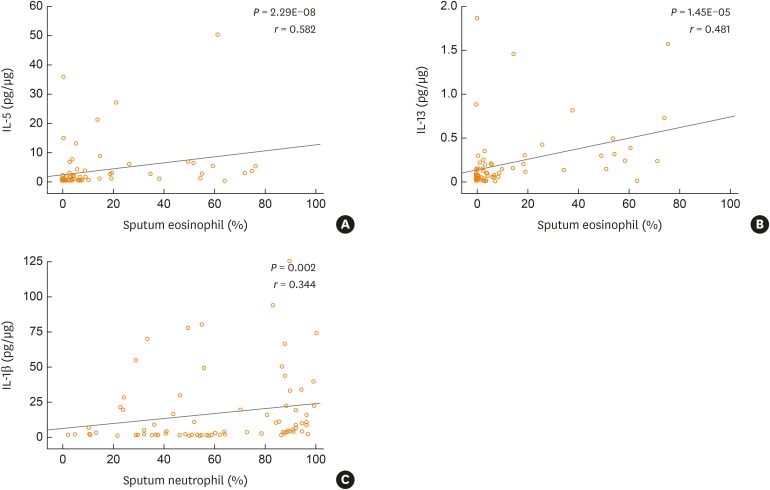
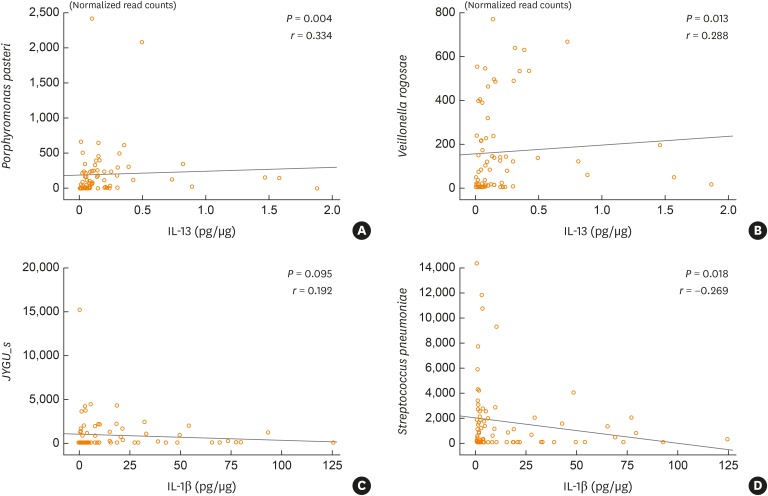
The numbers of operational taxonomic units were significantly higher in the mixed (n = 21) and neutrophilic (n = 23) inflammation groups than in the paucigranulocytic inflammation group (n = 19; p < 0.05). At the species level, Granulicatella adiacens, Streptococcus parasanguinis, Streptococcus pneumoniae, Veillonella rogosae, Haemophilus parainfluenzae, and Neisseria perflava levels were significantly higher in the eosinophilic inflammation group (n = 20), whereas JYGU_s levels were significantly higher in the neutrophilic inflammation group compared to the other subtypes (P < 0.05). Additionally, IL-5 and IL-13 concentrations were correlated with the percentage of eosinophils (P < 0.05) and IL-13 levels were positively correlated with the read counts of Porphyromonas pasteri and V. rogosae (P < 0.05). IL-1β concentrations were correlated with the percentage of neutrophils (P < 0.05). had a tendency to be positively correlated with the read count of JYGU_s (P = 0.095), and was negatively correlated with that of S. pneumoniae (P < 0.05).
CONCLUSIONS
Difference of microbial patterns in airways may induce distinctive endotypes of asthma, which is responsible for the neutrophilic or eosinophilic inflammation in asthma.
Keyword
MeSH Terms
-
Asthma
Colony-Stimulating Factors
Cytokines
Enzyme-Linked Immunosorbent Assay
Eosinophils*
Haemophilus parainfluenzae
Inflammasomes
Inflammation*
Interleukin-13
Interleukin-33
Interleukin-5
Microbiota
Necrosis
Neisseria
Neutrophils*
Phenotype
Pneumonia
Porphyromonas
Sequence Analysis
Sputum
Streptococcus
Streptococcus pneumoniae
Veillonella
Colony-Stimulating Factors
Cytokines
Inflammasomes
Interleukin-13
Interleukin-33
Interleukin-5
Figure
Cited by 1 articles
-
Does the Difference in Microbial Patterns in the Airways Induce Distinct Endotypes of Asthma?
Young Joo Cho
Allergy Asthma Immunol Res. 2020;12(3):375-377. doi: 10.4168/aair.2020.12.3.375.
Reference
-
1. Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006; 11:54–61. PMID: 16423202.
Article2. Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009; 6:256–259. PMID: 19387026.
Article3. Choi JS, Jang AS, Park JS, Park SW, Paik SH, Park JS, et al. Role of neutrophils in persistent airway obstruction due to refractory asthma. Respirology. 2012; 17:322–329. PMID: 22040093.
Article4. Pavord ID, Birring SS, Berry M, Green RH, Brightling CE, Wardlaw AJ. Multiple inflammatory hits and the pathogenesis of severe airway disease. Eur Respir J. 2006; 27:884–888. PMID: 16707390.5. Reed CE, Milton DK. Endotoxin-stimulated innate immunity: a contributing factor for asthma. J Allergy Clin Immunol. 2001; 108:157–166. PMID: 11496229.
Article6. Clapp WD, Becker S, Quay J, Watt JL, Thorne PS, Frees KL, et al. Grain dust-induced airflow obstruction and inflammation of the lower respiratory tract. Am J Respir Crit Care Med. 1994; 150:611–617. PMID: 8087327.
Article7. Kuschner WG, D'Alessandro A, Wong H, Blanc PD. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J. 1996; 9:1989–1994. PMID: 8902455.
Article8. Essilfie AT, Simpson JL, Dunkley ML, Morgan LC, Oliver BG, Gibson PG, et al. Combined Haemophilus influenzae respiratory infection and allergic airways disease drives chronic infection and features of neutrophilic asthma. Thorax. 2012; 67:588–599. PMID: 22387445.9. Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014; 9:e100645. PMID: 24955983.
Article10. Simpson JL, Daly J, Baines KJ, Yang IA, Upham JW, Reynolds PN, et al. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J. 2016; 47:792–800. PMID: 26647445.11. Taylor SL, Leong LE, Choo JM, Wesselingh S, Yang IA, Upham JW, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol. 2018; 141:94–103.e15. PMID: 28479329.
Article12. Gauvreau GM, Ellis AK, Denburg JA. Haemopoietic processes in allergic disease: eosinophil/basophil development. Clin Exp Allergy. 2009; 39:1297–1306. PMID: 19622087.
Article13. Brightling CE. Eosinophils, bronchitis and asthma: pathogenesis of cough and airflow obstruction. Pulm Pharmacol Ther. 2011; 24:324–327. PMID: 21074631.
Article14. Besnard AG, Togbe D, Couillin I, Tan Z, Zheng SG, Erard F, et al. Inflammasome-IL-1-Th17 response in allergic lung inflammation. J Mol Cell Biol. 2012; 4:3–10. PMID: 22147847.
Article15. Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 2014; 43:1067–1076. PMID: 24136334.
Article16. Manni ML, Trudeau JB, Scheller EV, Mandalapu S, Elloso MM, Kolls JK, et al. The complex relationship between inflammation and lung function in severe asthma. Mucosal Immunol. 2014; 7:1186–1198. PMID: 24549277.
Article17. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006; 6:173–182. PMID: 16498448.
Article18. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004; 303:1532–1535. PMID: 15001782.
Article19. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald JM, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008; 31:143–178. PMID: 18166595.
Article20. Park SW, Lee YM, Jang AS, Lee JH, Hwangbo Y, Kim DJ, et al. Development of chronic airway obstruction in patients with eosinophilic bronchitis: a prospective follow-up study. Chest. 2004; 125:1998–2004. PMID: 15189914.21. Herlemann DP, Labrenz M, Jürgens K, Bertilsson S, Waniek JJ, Andersson AF. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011; 5:1571–1579. PMID: 21472016.
Article22. Hur M, Kim Y, Song HR, Kim JM, Choi YI, Yi H. Effect of genetically modified poplars on soil microbial communities during the phytoremediation of waste mine tailings. Appl Environ Microbiol. 2011; 77:7611–7619. PMID: 21890678.
Article23. Janda JM, Abbott SL. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol. 2007; 45:2761–2764. PMID: 17626177.
Article24. Rogers GB, Hoffman LR, Carroll MP, Bruce KD. Interpreting infective microbiota: the importance of an ecological perspective. Trends Microbiol. 2013; 21:271–276. PMID: 23598051.
Article25. Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013; 131:346–352.e1-3. PMID: 23265859.
Article26. Park H, Shin JW, Park SG, Kim W. Microbial communities in the upper respiratory tract of patients with asthma and chronic obstructive pulmonary disease. PLoS One. 2014; 9:e109710. PMID: 25329665.
Article27. Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010; 5:e8578. PMID: 20052417.
Article28. Pérez-Losada M, Castro-Nallar E, Bendall ML, Freishtat RJ, Crandall KA. Dual transcriptomic profiling of host and microbiota during health and disease in pediatric asthma. PLoS One. 2015; 10:e0131819. PMID: 26125632.
Article29. Goleva E, Jackson LP, Harris JK, Robertson CE, Sutherland ER, Hall CF, et al. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med. 2013; 188:1193–1201. PMID: 24024497.
Article30. Pang Z, Wang G, Gibson P, Guan X, Zhang W, Zheng R, et al. Airway microbiome in different inflammatory phenotypes of asthma: a cross-sectional study in Northeast China. Int J Med Sci. 2019; 16:477–485. PMID: 30911282.
Article31. Yang X, Li H, Ma Q, Zhang Q, Wang C. Neutrophilic asthma is associated with increased airway bacterial burden and disordered community composition. BioMed Res Int. 2018; 2018:9230234. PMID: 30105264.
Article32. Tliba O, Panettieri RA Jr. Paucigranulocytic asthma: uncoupling of airway obstruction from inflammation. J Allergy Clin Immunol. 2019; 143:1287–1294. PMID: 29928921.
Article33. Hirata SI, Kunisawa J. Gut microbiome, metabolome, and allergic diseases. Allergol Int. 2017; 66:523–528. PMID: 28693971.
Article34. ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med. 2001; 164:744–748. PMID: 11549526.
Article35. Jo WK, Kim KY, Park KH, Kim YK, Lee HW, Park JK. Comparison of outdoor and indoor mobile source-related volatile organic compounds between low- and high-floor apartments. Environ Res. 2003; 92:166–171. PMID: 12854697.
Article36. Maliszewska-Kordybach B. Sources, concentrations, fate and effects of polycyclic aromatic hydrocarbons (PAHs) in the environment. Part A: PAHs in air. Pol J Environ Stud. 1999; 8:131–136.37. Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY, Park J, et al. Extracellular vesicles, especially derived from Gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin Exp Allergy. 2013; 43:443–454. PMID: 23517040.
Article38. Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007; 120:1279–1284. PMID: 17949801.39. Hams E, Fallon PG. Innate type 2 cells and asthma. Curr Opin Pharmacol. 2012; 12:503–509. PMID: 22749407.
Article40. Jakubovics NS, Strömberg N, van Dolleweerd CJ, Kelly CG, Jenkinson HF. Differential binding specificities of oral streptococcal antigen I/II family adhesins for human or bacterial ligands. Mol Microbiol. 2005; 55:1591–1605. PMID: 15720563.
Article41. Hansel TT, Johnston SL, Openshaw PJ. Microbes and mucosal immune responses in asthma. Lancet. 2013; 381:861–873. PMID: 23428115.
Article42. de Vries SP, Eleveld MJ, Hermans PW, Bootsma HJ. Characterization of the molecular interplay between Moraxella catarrhalis and human respiratory tract epithelial cells. PLoS One. 2013; 8:e72193. PMID: 23936538.43. Su YC, Singh B, Riesbeck K. Moraxella catarrhalis: from interactions with the host immune system to vaccine development. Future Microbiol. 2012; 7:1073–1100. PMID: 22953708.44. Slevogt H, Seybold J, Tiwari KN, Hocke AC, Jonatat C, Dietel S, et al. Moraxella catarrhalis is internalized in respiratory epithelial cells by a trigger-like mechanism and initiates a TLR2- and partly NOD1-dependent inflammatory immune response. Cell Microbiol. 2007; 9:694–707. PMID: 17054439.
Article45. Veiga-Malta I, Duarte M, Dinis M, Tavares D, Videira A, Ferreira P. Enolase from Streptococcus sobrinus is an immunosuppressive protein. Cell Microbiol. 2004; 6:79–88. PMID: 14678332.46. Otero C, Paz RD, Galassi N, Bezrodnik L, Finiasz MR, Fink S. Immune response to Streptococcus pneumoniae in asthma patients: comparison between stable situation and exacerbation. Clin Exp Immunol. 2013; 173:92–101. PMID: 23607482.47. Miki-Hosokawa T, Hasegawa A, Iwamura C, Shinoda K, Tofukuji S, Watanabe Y, et al. CD69 controls the pathogenesis of allergic airway inflammation. J Immunol. 2009; 183:8203–8215. PMID: 19923457.
Article48. Martín P, Sánchez-Madrid F. CD69: an unexpected regulator of TH17 cell-driven inflammatory responses. Sci Signal. 2011; 4:pe14. PMID: 21427408.49. Karched M, Bhardwaj RG, Tiss A, Asikainen S. Proteomic analysis and virulence assessment of Granulicatella adiacens secretome. Front Cell Infect Microbiol. 2019; 9:104. PMID: 31069174.
Article50. Reis AC, Alessandri AL, Athayde RM, Perez DA, Vago JP, Ávila TV, et al. Induction of eosinophil apoptosis by hydrogen peroxide promotes the resolution of allergic inflammation. Cell Death Dis. 2015; 6:e1632. PMID: 25675292.
Article51. Erzurum SC. New insights in oxidant biology in asthma. Ann Am Thorac Soc. 2016; 13 Suppl 1:S35–S39. PMID: 27027950.52. Simpson JL, Carroll M, Yang IA, Reynolds PN, Hodge S, James AL, et al. Reduced antiviral interferon production in poorly controlled asthma is associated with neutrophilic inflammation and high-dose inhaled corticosteroids. Chest. 2016; 149:704–713. PMID: 26836898.
Article53. Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000; 2:1599–1607. PMID: 11113379.
Article54. Delwiche EA, Pestka JJ, Tortorello ML. The Veillonellae: Gram-negative cocci with a unique physiology. Annu Rev Microbiol. 1985; 39:175–193. PMID: 3904599.55. Kara D, Luppens SB, Cate JM. Differences between single- and dual-species biofilms of Streptococcus mutans and Veillonella parvula in growth, acidogenicity and susceptibility to chlorhexidine. Eur J Oral Sci. 2006; 114:58–63. PMID: 16460342.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune Cell-Mediated Autoimmune Responses in Severe Asthma
- Effect of Coexistence of Atopic Dermatitis on Eosinophilic Lower Airway Inflammation in Mild Persistent Asthma
- The Effect of Atopy and Airway Eosinophilic Inflammation on Exercise-Induced Bronchospasm in Asthmatics
- Airway epithelial cells in airway inflammation and remodeling in asthma
- IL-13 And IL-5 in Induced Sputum in Patients with Eosinophilic Bronchitis and Asthma: an Association with Airway Hyperresponsiveness