J Breast Cancer.
2019 Dec;22(4):624-634. 10.4048/jbc.2019.22.e53.
Amenorrhea and Menopause in Patients with Breast Cancer after Chemotherapy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. ymchoi@snu.ac.kr
- 2Fertility Center, Heryoojae Women's Hospital, Goyang, Korea.
- 3Medical Research Center, the Institute of Reproductive Medicine and Population, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 5Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2470898
- DOI: http://doi.org/10.4048/jbc.2019.22.e53
Abstract
- PURPOSE
The probability of ovarian failure after cytotoxic chemotherapy in patients with breast cancer has not been well established in Korea. This study aimed to assess the rate of ovarian failure in a large cohort of Korean premenopausal patients with breast cancer 12 months after chemotherapy.
METHODS
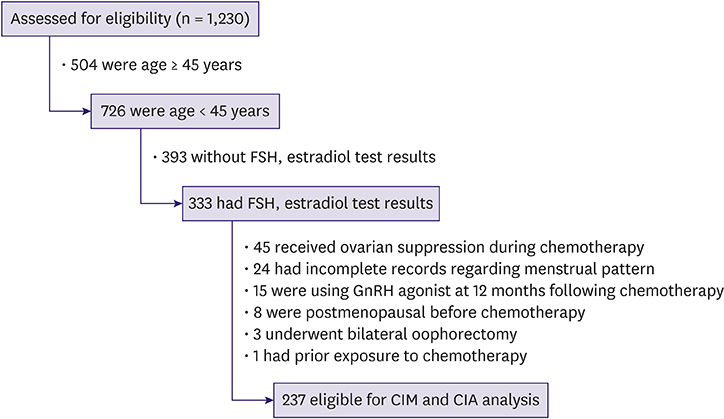
This retrospective cohort study included premenopausal women (aged 20−44 years) with breast cancer who underwent chemotherapy after surgery. The rates of treatment-related amenorrhea (TRA) and chemotherapy-induced menopause (CIM) at 12 months after chemotherapy were analyzed.
RESULTS
A total of 237 patients met the inclusion criteria. The rate of TRA was 61.6% and that of CIM was 13.1% at 12 months after chemotherapy. The rates of TRA and CIM were 28.0% and 4.0%, respectively, in women aged 25−34 years, and they gradually increased up to 75.9% (TRA) and 15.8% (CIM), respectively, in women aged 40−44 years. The frequency of CIM was significantly lower than that of TRA in both age groups. In multivariate analyses, only tamoxifen use was significantly associated with a decreased risk of CIM (p < 0.001). Age of 40 years or higher and the regimens of doxorubicin plus cyclophosphamide followed by docetaxel or paclitaxel were associated with increased risk of TRA (p = 0.001 and p = 0.002, respectively).
CONCLUSION
Marked discrepancy in the rates of CIM and TRA was observed in this study. Further, the age-specific frequency of CIM and TRA observed in this study is a reliable and practical estimate of the risks of CIM and TRA in the absence of gonadal protection.
Keyword
MeSH Terms
Figure
Reference
-
1. Park EH, Min SY, Kim Z, Yoon CS, Jung KW, Nam SJ, et al. Basic facts of breast cancer in Korea in 2014: the 10-year overall survival progress. J Breast Cancer. 2017; 20:1–11.
Article2. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017; 49:292–305.
Article3. Breast cancer facts & figures, 2015–2016. American Cancer Society. Accessed September 1, 2016. https://www.cancer.org/research/cancerfactsstatistics/acspc-046381.4. Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009; 4:e7695.
Article5. Yu B, Douglas N, Ferin MJ, Nakhuda GS, Crew K, Lobo RA, et al. Changes in markers of ovarian reserve and endocrine function in young women with breast cancer undergoing adjuvant chemotherapy. Cancer. 2010; 116:2099–2105.
Article6. Brydøy M, Fosså SD, Dahl O, Bjøro T. Gonadal dysfunction and fertility problems in cancer survivors. Acta Oncol. 2007; 46:480–489.
Article7. NCCN clinical practice guidelines in oncology. Breast cancer. National Comprehensive Cancer Network (US). Accessed October 2, 2019. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.8. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015; 22:3230–3235.
Article9. Del Mastro L, Boni L, Michelotti A, Gamucci T, Olmeo N, Gori S, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011; 306:269–276.10. Song G, Gao H, Yuan Z. Effect of leuprolide acetate on ovarian function after cyclophosphamide-doxorubicin-based chemotherapy in premenopausal patients with breast cancer: results from a phase II randomized trial. Med Oncol. 2013; 30:667.
Article11. Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009; 91:694–697.
Article12. Moore HC, Unger JM, Phillips KA, Boyle F, Hitre E, Porter D, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015; 372:923–932.
Article13. Tiong V, Rozita AM, Taib NA, Yip CH, Ng CH. Incidence of chemotherapy-induced ovarian failure in premenopausal women undergoing chemotherapy for breast cancer. World J Surg. 2014; 38:2288–2296.
Article14. Torino F, Barnabei A, De Vecchis L, Appetecchia M, Strigari L, Corsello SM. Recognizing menopause in women with amenorrhea induced by cytotoxic chemotherapy for endocrine-responsive early breast cancer. Endocr Relat Cancer. 2012; 19:R21–33.
Article15. Clemons M, Simmons C. Identifying menopause in breast cancer patients: considerations and implications. Breast Cancer Res Treat. 2007; 104:115–120.
Article16. Lambalk CB, van Disseldorp J, de Koning CH, Broekmans FJ. Testing ovarian reserve to predict age at menopause. Maturitas. 2009; 63:280–291.
Article17. Demeestere I, Brice P, Peccatori FA, Kentos A, Gaillard I, Zachee P, et al. Gonadotropin-releasing hormone agonist for the prevention of chemotherapy-induced ovarian failure in patients with lymphoma: 1-year follow-up of a prospective randomized trial. J Clin Oncol. 2013; 31:903–909.
Article18. Zavos A, Valachis A. Risk of chemotherapy-induced amenorrhea in patients with breast cancer: a systematic review and meta-analysis. Acta Oncol. 2016; 55:664–670.
Article19. Han HS, Ro J, Lee KS, Nam BH, Seo JA, Lee DH, et al. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat. 2009; 115:335–342.
Article20. Park IH, Han HS, Lee H, Lee KS, Kang HS, Lee S, et al. Resumption or persistence of menstruation after cytotoxic chemotherapy is a prognostic factor for poor disease-free survival in premenopausal patients with early breast cancer. Ann Oncol. 2012; 23:2283–2289.
Article21. Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006; 24:1045–1051.
Article22. Vital-Reyes V, Tellez-Velasco S, Chhieng D, Grizzle W, Reyes-Fuentes A. Spontaneous pregnancy in a woman with premature ovarian failure: a case report. J Reprod Med. 2004; 49:989–991.23. Partridge AH, Ruddy KJ, Gelber S, Schapira L, Abusief M, Meyer M, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010; 94:638–644.
Article24. Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, et al. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012; 97:134–140.e1.
Article25. Chien AJ, Duralde E, Hwang R, Tsung K, Kao CN, Rugo HS, et al. Association of tamoxifen use and ovarian function in patients with invasive or pre-invasive breast cancer. Breast Cancer Res Treat. 2015; 153:173–181.
Article26. Jordan VC, Fritz NF, Tormey DC. Endocrine effects of adjuvant chemotherapy and long-term tamoxifen administration on node-positive patients with breast cancer. Cancer Res. 1987; 47:624–630.27. Ravdin PM, Fritz NF, Tormey DC, Jordan VC. Endocrine status of premenopausal node-positive breast cancer patients following adjuvant chemotherapy and long-term tamoxifen. Cancer Res. 1988; 48:1026–1029.28. Jung M, Shin HJ, Rha SY, Jeung HC, Hong S, Moon YW, et al. The clinical outcome of chemotherapy-induced amenorrhea in premenopausal young patients with breast cancer with long-term follow-up. Ann Surg Oncol. 2010; 17:3259–3268.
Article29. Liem GS, Mo FK, Pang E, Suen JJ, Tang NL, Lee KM, et al. Chemotherapy-related amenorrhea and menopause in young Chinese breast cancer patients: analysis on incidence, risk factors and serum hormone profiles. PLoS One. 2015; 10:e0140842.
Article30. Fornier MN, Modi S, Panageas KS, Norton L, Hudis C. Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer. 2005; 104:1575–1579.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Incidence of Chemotherapy-induced Amenorrhea and Recovery in Young (<45-year-old) Breast Cancer Patients
- Factors of Occurrence of Amenorrhea and Climacteric Symptoms in Breast Cancer Patients Underwent Chemotherapy
- Menopausal Symptoms and Quality of Life Among Breast Cancer Patients with Chemotherapy-induced Amenorrhea
- Recovery of Ovarian Function with Aromatase Inhibitors: In Young Breast Cancer Patients (<45) with Chemotherapy-induced Amenorrhea
- The Effect of Mirtazapine for Treatment of Hot Flashes in Depressed Woman with Breast Cancer Receiving Tamoxifen: A Case Report