J Korean Med Sci.
2016 Nov;31(11):1726-1734. 10.3346/jkms.2016.31.11.1726.
Neurocognitive Function and Health-Related Quality of Life in Pediatric Korean Survivors of Medulloblastoma
- Affiliations
-
- 1Department of Neurosurgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 2Department of Pediatrics, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 3Center for Pediatric Cancer, National Cancer Center, Goyang, Korea. hjpark@ncc.re.kr
- 4Department of Pediatric Neurosurgery, Severance Children’s Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Pediatrics, Seoul National University Children's Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2470267
- DOI: http://doi.org/10.3346/jkms.2016.31.11.1726
Abstract
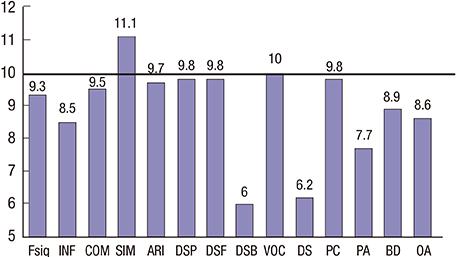
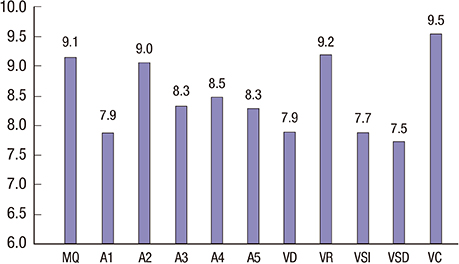
- The neurocognitive function and quality of life of 58 Korean survivors of childhood medulloblastoma were assessed after surgery, cranial radiation and chemotherapy. All patients were evaluated with a battery of neurocognitive function tests and the Pediatric Functional Assessment of Cancer Therapy-Brain Tumor Survivors, which consists of self-report questionnaires on quality of life. The mean full-scale intelligence quotient (IQ), verbal IQ, and performance IQ scores were 90.2, 97.1, and 84.16, respectively. The mean memory quotient (MQ) score was 86.78, which was within 1 standard deviation of the average score of 100. Processing speed, attention, and executive function showed mild to moderate deficits. Intelligence, memory, executive function, visuospatial function, and simple motor function were significantly lower in the patients diagnosed before 8 years of age compared with those diagnosed after 8. The cognitive deficits in the patients diagnosed at younger ages might be related to earlier exposure to craniospinal irradiation and chemotherapy. The patient and parent proxy evaluations of attention, fine motor function, and quality of life did not differ. We found significant neurocognitive changes in a wide range of neurocognitive functional domains in Korean survivors of childhood medulloblastoma. Long-term follow-up studies of survivors of childhood medulloblastoma beginning at the time of their first diagnosis are required to better understand the deficits exhibited by survivors of childhood medulloblastoma, so that intervention strategies and treatment refinements that reduce the long-term neurocognitive decline can be developed.
Keyword
MeSH Terms
-
Adolescent
Adult
Asian Continental Ancestry Group
Attention
Cerebellar Neoplasms/*physiopathology/radiotherapy/surgery
Child
Child, Preschool
Cognition
Cranial Irradiation
Female
Follow-Up Studies
Humans
Infant
Intelligence Tests
Male
Medulloblastoma/*physiopathology/radiotherapy/surgery
Memory
Neuropsychological Tests
*Quality of Life
Radiation Dosage
Republic of Korea
Surveys and Questionnaires
Survivors/psychology
Young Adult
Figure
Cited by 1 articles
-
Risk Stratification of Childhood Medulloblastoma Using Integrated Diagnosis: Discrepancies with Clinical Risk Stratification
Hee Won Cho, Hyunwoo Lee, Hee Young Ju, Keon Hee Yoo, Hong Hoe Koo, Do Hoon Lim, Ki Woong Sung, Hyung Jin Shin, Yeon-Lim Suh, Ji Won Lee
J Korean Med Sci. 2022;37(7):e59. doi: 10.3346/jkms.2022.37.e59.
Reference
-
1. Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004; 22:706–713.2. Kieffer-Renaux V, Viguier D, Raquin MA, Laurent-Vannier A, Habrand JL, Dellatolas G, Kalifa C, Hartmann O, Grill J. Therapeutic schedules influence the pattern of intellectual decline after irradiation of posterior fossa tumors. Pediatr Blood Cancer. 2005; 45:814–819.3. Khong PL, Leung LH, Fung AS, Fong DY, Qiu D, Kwong DL, Ooi GC, McAlonan G, Cao G, Chan GC. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006; 24:884–890.4. Rønning C, Sundet K, Due-Tønnessen B, Lundar T, Helseth E. Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumors in childhood. Pediatr Neurosurg. 2005; 41:15–21.5. Maddrey AM, Bergeron JA, Lombardo ER, McDonald NK, Mulne AF, Barenberg PD, Bowers DC. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J Neurooncol. 2005; 72:245–253.6. George AP, Kuehn SM, Vassilyadi M, Richards PM, Parlow SE, Keene DL, Ventureyra EC. Cognitive sequelae in children with posterior fossa tumors. Pediatr Neurol. 2003; 28:42–47.7. Reeves CB, Palmer SL, Reddick WE, Merchant TE, Buchanan GM, Gajjar A, Mulhern RK. Attention and memory functioning among pediatric patients with medulloblastoma. J Pediatr Psychol. 2006; 31:272–280.8. Bhat SR, Goodwin TL, Burwinkle TM, Lansdale MF, Dahl GV, Huhn SL, Gibbs IC, Donaldson SS, Rosenblum RK, Varni JW, et al. Profile of daily life in children with brain tumors: an assessment of health-related quality of life. J Clin Oncol. 2005; 23:5493–5500.9. Park HJ, Nam BH, Lim HS, Shin HY, Hah JO, Kang HJ, Koo HH, Kook H, Kim DS, Kim SK, et al. Outcome of multicenter study for Korean children with medulloblastoma. Clin Pediatr Hematol Oncol. 2007; 14:167–175.10. Yum TH, Park YS, Oh KJ, Kim JG, Lee HY. The Manual of Korean-Wechsler Adult Intelligence Scale. Seoul: Korean Guidance Press;1992.11. Parekh PI, Blumenthal JA, Babyak MA, LaCaille R, Rowe S, Dancel L, Carney RM, Davis RD, Palmer S. INSPIRE Investigators. Gas exchange and exercise capacity affect neurocognitive performance in patients with lung disease. Psychosom Med. 2005; 67:425–432.12. Shin MS, Goo HJ. Children's Color Trails Test. Seoul: Hakjisa;2007.13. Williams J, Rickert V, Hogan J, Zolten AJ, Satz P, D’Elia LF, Asarnow RF, Zaucha K, Light R. Children’s color trails. Arch Clin Neuropsychol. 1995; 10:211–223.14. Kim HG. Rey-Kim Memory Test. Daegu: Neuropsychology Press;1999.15. Lezak MD. Neuropsychological Assessment. 2nd ed. New York, NY: Oxford University;1983.16. Mitrushina MN, Boone KB, D'Elia LF. Handbook of Normative Data for Neuropsychological Assessment. New York, NY: Oxford University Press;1999.17. Kwak GJ, Park HW, Kim CT. Korean Wechsler Intelligence Scale for Children-III. Seoul: Special Education Publishing Co.;2001.18. Ferrans CE. Development of a quality of life index for patients with cancer. Oncol Nurs Forum. 1990; 17:15–19.19. Cella D. FACIT Manual: Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System. Evanston, IL: Center on Outcomes, Research and Education, Evanston Northwestern Healthcare and Northwestern University;1997.20. Yoo H, Ra YS, Park HJ, Lai JS, Cella D, Shin HY, Kim DS, Kim WC, Shin YS. Validation of pediatric functional assessment of cancer therapy: patient version 2 of “brain tumor survivor” for grade school patients aged 7-12 years. Qual Life Res. 2011; 20:529–535.21. Yoo H, Kim DS, Shin HY, Lai JS, Cella D, Park HJ, Ra YS, Kim WC, Shin YS. Validation of the pediatric functional assessment of cancer therapy questionnaire (version 2.0) in brain tumor survivors aged 13 years and older. J Pain Symptom Manage. 2010; 40:559–565.22. Shin HY. Korean society for pediatric neuro-oncology (KSPNO). Korean J Pediatr Hematol Oncol. 2005; 12:175–187.23. Pendergrass TW, Milstein JM, Geyer JR, Mulne AF, Kosnik EJ, Morris JD, Heideman RL, Ruymann FB, Stuntz JT, Bleyer WA. Eight drugs in one day chemotherapy for brain tumors: experience in 107 children and rationale for preradiation chemotherapy. J Clin Oncol. 1987; 5:1221–1231.24. Tarbell NJ, Friedman H, Polkinghorn WR, Yock T, Zhou T, Chen Z, Burger P, Barnes P, Kun L. High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol. 2013; 31:2936–2941.25. Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006; 24:4202–4208.26. Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L, Merchant TE, Mulhern RK. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001; 19:2302–2308.27. Moxon-Emre I, Bouffet E, Taylor MD, Laperriere N, Scantlebury N, Law N, Spiegler BJ, Malkin D, Janzen L, Mabbott D. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014; 32:1760–1768.28. Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a children’s cancer group study. J Clin Oncol. 2001; 19:3470–3476.29. Tulsky DS, Saklofske DH, Zhu J. Revising a Standard: an Evaluation of the Origin and Development of the WAIS-III. San Diego, CA: Academic Press;2003.30. Lai JS, Cella D, Tomita T, Bode RK, Newmark M, Goldman S. Developing a health-related quality of life instrument for childhood brain tumor survivors. Childs Nerv Syst. 2007; 23:47–57.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Health-related Needs and Quality of Life in Childhood Cancer Survivors
- Health-Related Quality of Life in Breast Cancer Patients with Lymphedema Who Survived More than One Year after Surgery
- Gonadal Function in Female Childhood Cancer Survivors
- Impact of Poststroke Fatigue on Health-Related Quality of Life of Nigerian Stroke Survivors
- Distress and Quality of Life in Breast Cancer Survivors in Korea