J Korean Acad Prosthodont.
2020 Jan;58(1):14-22. 10.4047/jkap.2020.58.1.14.
The biofilm removal effect of MnOâ‚‚-diatom microbubbler from the dental prosthetic surfaces: In vitro study
- Affiliations
-
- 1Department of Prosthodontics, School of Dentistry, Seoul National University, Seoul, Republic of Korea. silk1@snu.ac.kr
- 2Department of Chemical and Biomolecular Engineering, University of Illinois, Urbana-Champaign, Urbana, IL, USA.
- KMID: 2469916
- DOI: http://doi.org/10.4047/jkap.2020.58.1.14
Abstract
- PURPOSE
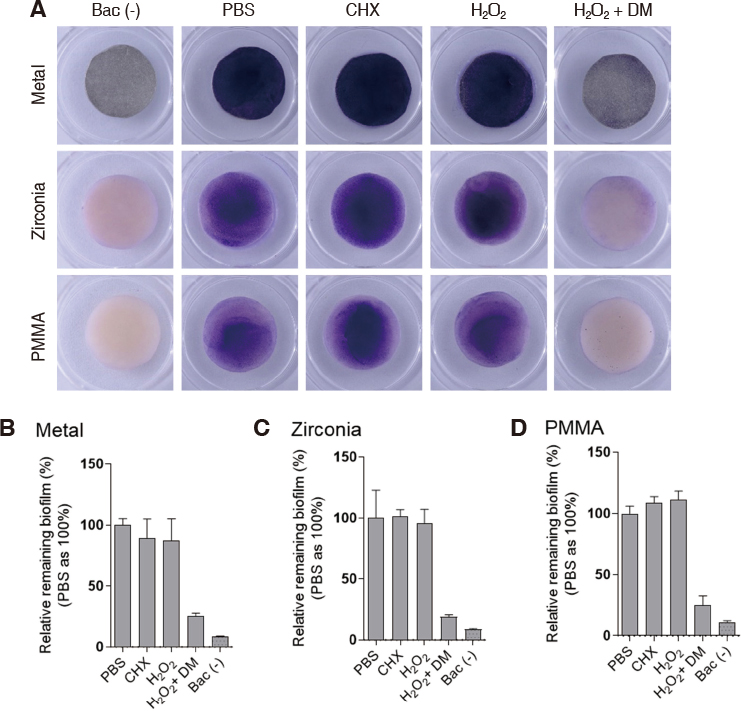
The aim of this study is to evaluate the effectiveness of MnOâ‚‚-diatom microbubbler (DM) on the surface of prosthetic materials as a mouthwash by comparing the biofilm removal effect with those previously used as a mouthwash in dental clinic.
MATERIALS AND METHODS
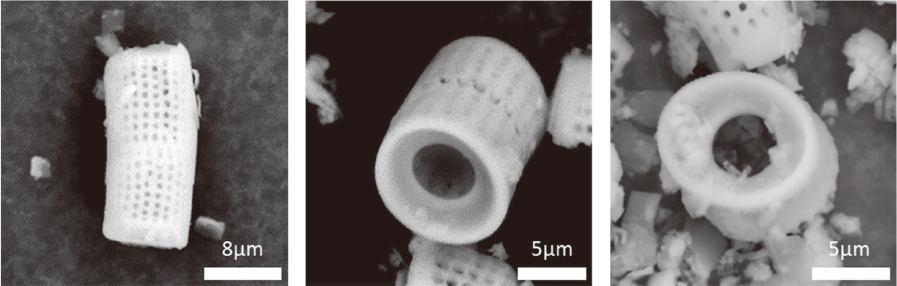
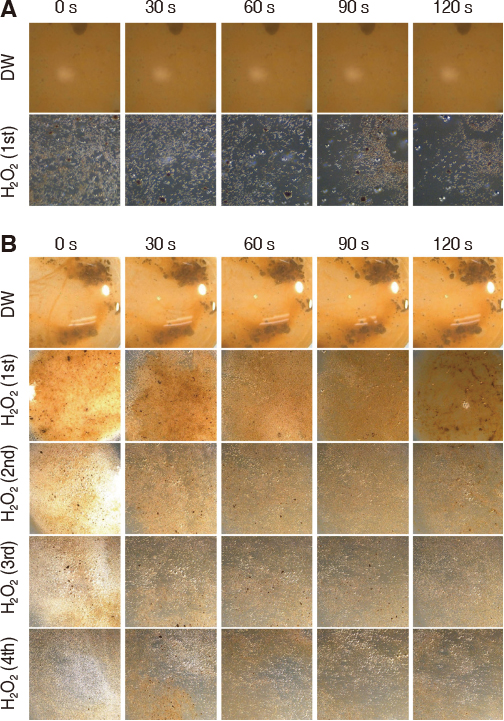
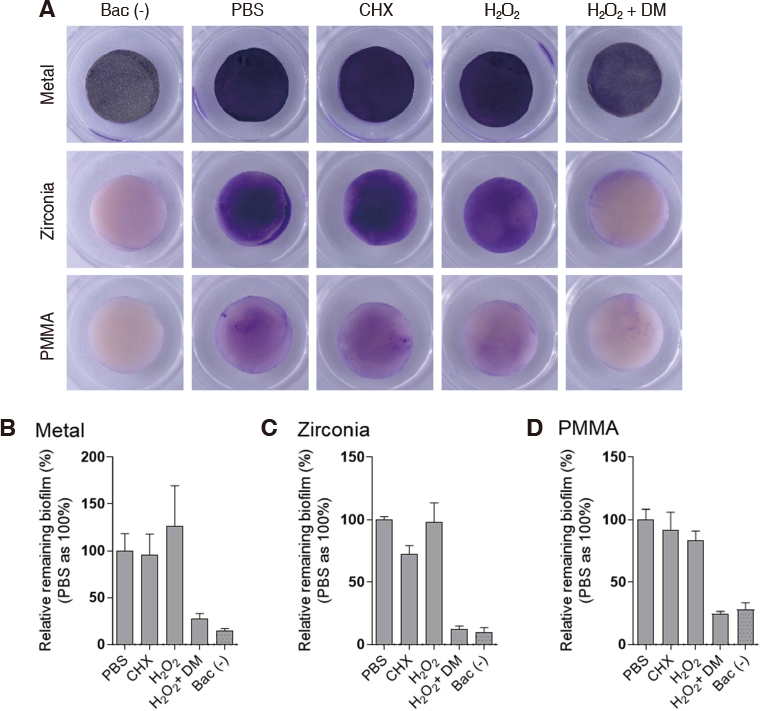
DM was fabricated by doping manganese dioxide nanosheets to the diatom cylinder surface. Scanning electron microscopy (SEM) was used to observe the morphology of DM and to analyze the composition of doped MnOâ‚‚. Stereomicroscope was used to observe the reaction of DM in 3% hydrogen peroxide. Non-precious metal alloys, zirconia and resin specimens were prepared to evaluate the effect of biofilm removal on the surface of prosthetic materials. And then Streptococcus mutans and Porphyromonas gingivalis biofilms were formed on the specimens. When 3% hydrogen peroxide solution and DM were treated on the biofilms, the decontamination effect was compared with chlorhexidine gluconate and 3% hydrogen peroxide solution by crystal violet staining.
RESULTS
Manganese dioxide was found on the surface of the diatom cylinder, and it was found to produce bubble of oxygen gas when added to 3% hydrogen peroxide. For all materials used in the experiments, biofilms of the DM-treated groups got effectively removed compared to the groups used with chlorhexidine gluconate or 3% hydrogen peroxide alone.
CONCLUSION
MnOâ‚‚-diatom microbubbler can remove bacterial membranes on the surface of prosthetic materials more effectively than conventional mouthwashes.
MeSH Terms
-
Alloys
Biofilms*
Chlorhexidine
Decontamination
Dental Clinics
Dental Plaque
Diatoms
Gentian Violet
Hydrogen Peroxide
In Vitro Techniques*
Manganese
Membranes
Microscopy, Electron, Scanning
Mouthwashes
Oral Hygiene
Oxygen
Porphyromonas gingivalis
Streptococcus mutans
Alloys
Chlorhexidine
Gentian Violet
Hydrogen Peroxide
Manganese
Mouthwashes
Oxygen
Figure
Reference
-
1. Thomas JG, Nakaishi LA. Managing the complexity of a dynamic biofilm. J Am Dent Assoc. 2006; 137:10S–15S.
Article2. Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979; 6:351–382.
Article3. Axelsson P, Lindhe J. The effect of a preventive programme on dental plaque, gingivitis and caries in schoolchildren. Results after one and two years. J Clin Periodontol. 1974; 1:126–138.
Article4. McKendrick AJ, Barbenel MH, McHugh WD. The influence of time of examination, eating, smoking and frequency of brushing on the oral debris index. J Periodontal Res. 1970; 5:205–207.
Article5. Binney A, Addy M, Newcombe RG. The effect of a number of commercial mouthrinses compared with toothpaste on plaque regrowth. J Periodontol. 1992; 63:839–842.
Article6. Wu CD, Savitt ED. Evaluation of the safety and efficacy of over-the-counter oral hygiene products for the reduction and control of plaque and gingivitis. Periodontol 2000. 2002; 28:91–105.
Article7. Gunsolley JC. Clinical efficacy of antimicrobial mouthrinses. J Dent. 2010; 38:S6–S10.
Article8. Barnett ML. The role of therapeutic antimicrobial mouthrinses in clinical practice: control of supragingival plaque and gingivitis. J Am Dent Assoc. 2003; 134:699–704.9. Löe H, Schiott CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970; 5:79–83.
Article10. Addy M, Jenkins S, Newcombe R. The effect of some chlorhexidine-containing mouthrinses on salivary bacterial counts. J Clin Periodontol. 1991; 18:90–93.
Article11. Grossman E, Meckel AH, Isaacs RL, Ferretti GA, Sturzenberger OP, Bollmer BW, Moore DJ, Lijana RC, Manhart MD. A clinical comparison of antibacterial mouthrinses: effects of chlorhexidine, phenolics, and sanguinarine on dental plaque and gingivitis. J Periodontol. 1989; 60:435–440.12. Santos A. Evidence-based control of plaque and gingivitis. J Clin Periodontol. 2003; 30:13–16.
Article13. Scheie AA. Modes of action of currently known chemical antiplaque agents other than chlorhexidine. J Dent Res. 1989; 68:1609–1616.14. Haskel E, Esquenasi J, Yussim L. Effects of subgingival chlorhexidine irrigation in chronic moderate periodontitis. J Periodontol. 1986; 57:305–310.
Article15. Seo Y, Leong J, Park JD, Hong YT, Chu SH, Park C, Kim DH, Deng YH, Dushnov V, Soh J, Rogers S, Yang YY, Kong HJ. Diatom microbubbler for active biofilm removal in confined spaces. ACS Appl Mater Interfaces. 2018; 10:35685–35692.
Article16. Sánchez MC, Llama-Palacios A, Blanc V, León R, Herrera D, Sanz M. Structure, viability and bacterial kinetics of an in vitro biofilm model using six bacteria from the subgingival microbiota. J Periodontal Res. 2011; 46:252–260.
Article17. Richmond S, Chestnutt I, Shennan J, Brown R. The relationship of medical and dental factors to perceived general and dental health. Community Dent Oral Epidemiol. 2007; 35:89–97.
Article18. Locker D, Matear D, Lawrence H. General health status and changes in chewing ability in older canadians over seven years. J Public Health Dent. 2002; 62:70–77.
Article19. Valderhaug J, Birkeland JM. Periodontal conditions in patients 5 years following insertion of fixed prostheses. Pocket depth and loss of attachment. J Oral Rehabil. 1976; 3:237–243.
Article20. Yoon JH, Park YB, Oh NS. Analysis of longevity and success rate of fixed, removable, and implant prostheses treated in Korea. J Korean Acad Prosthodont. 2018; 56:95–104.
Article21. Mandel ID. Chemotherapeutic agents for controlling plaque and gingivitis. J Clin Periodontol. 1988; 15:488–498.
Article22. Chaves ES, Kornman KS, Manwell MA, Jones AA, Newbold DA, Wood RC. Mechanism of irrigation effects on gingivitis. J Periodontol. 1994; 65:1016–1021.
Article23. Segreto VA, Collins EM, Beiswanger BB, de La Rosa M, Isaacs RL, Lang NP, Mallatt ME, Meckel AH. A comparison of mouthrinses containing two concentrations of chlorhexidine. J Periodontal Res. 1986; 21:23–32.
Article24. Grossman E, Reiter G, Sturzenberger OP, De La Rosa M, Dickinson TD, Flrretti GA, Ludlam GE, Meckel AH. Sixmonth study of the effects of a chlorhexidine mouthrinse on gingivitis in adults. J Periodontal Res. 1986; 21:33–43.
Article25. Badwey JA, Karnovsky ML. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980; 49:695–726.
Article26. Clifford DP, Repine JE. Hydrogen peroxide mediated killing of bacteria. Mol Cell Biochem. 1982; 49:143–149.
Article27. Brown MR, Gilbert P. Sensitivity of biofilms to antimicrobial agents. J Appl Bacteriol. 1993; 74:87S–97S.
Article28. Dostie S, Alkadi LT, Owen G, Bi J, Shen Y, Haapasalo M, Larjava HS. Chemotherapeutic decontamination of dental implants colonized by mature multispecies oral biofilm. J Clin Periodontol. 2017; 44:403–409.
Article29. Ikai H, Nakamura K, Shirato M, Kanno T, Iwasawa A, Sasaki K, Niwano Y, Kohno M. Photolysis of hydrogen peroxide, an effective disinfection system via hydroxyl radical formation. Antimicrob Agents Chemother. 2010; 54:5086–5091.
Article30. Wennström JL, Heijl L, Dahlén G, Gröndahl K. Periodic subgingival antimicrobial irrigation of periodontal pockets (I). Clinical observations. J Clin Periodontol. 1987; 14:541–550.
Article31. Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000; 131:887–899.
Article32. Mombelli A. Microbiology and antimicrobial therapy of periimplantitis. Periodontol 2000. 2002; 28:177–189.
Article33. Potempa J, Sroka A, Imamura T, Travis J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr Protein Pept Sci. 2003; 4:397–407.
Article34. Ahrari F, Eslami N, Rajabi O, Ghazvini K, Barati S. The antimicrobial sensitivity of Streptococcus mutans and Streptococcus sangius to colloidal solutions of different nanoparticles applied as mouthwashes. Dent Res J (Isfahan). 2015; 12:44–49.
Article35. Zahradnik RT, Magnusson I, Walker C, McDonell E, Hillman CH, Hillman JD. Preliminary assessment of safety and effectiveness in humans of ProBiora3, a probiotic mouthwash. J Appl Microbiol. 2009; 107:682–690.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: The biofilm removal effect of MnO2-diatom microbubbler fromthe dental prosthetic surfaces: In vitro study
- The Effects of Biofilm Care on Subgingival Bacterial Motility and Halitosis
- Progress in Method and Reliability of Diatom Test in Drowning
- An in vitro model of Fusobacterium nucleatum and Porphyromonas gingivalis in single- and dual-species biofilms
- Effect of cetylpyridinium chloride on dental unit waterline biofilms in-vitro