Cross-sectional analysis of immunosuppressive regimens focused on everolimus after liver transplantation in a Korean high-volume transplantation center
- Affiliations
-
- 1Division of Liver Transplantation and Hepatobiliary Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. shwang@amc.seoul.kr
- 2Department of Surgery, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- KMID: 2468159
- DOI: http://doi.org/10.4285/jkstn.2019.33.4.98
Abstract
- BACKGROUND
The mammalian target of the rapamycin inhibitor has dual inhibitory effects on cell growth and angiogenesis. This study aimed to analyze the usage of everolimus on actual immunosuppression (IS) regimens through a cross-sectional study in a high-volume liver transplantation (LT) center.
METHODS
Our institutional LT database was searched for adult patients who underwent primary LT surgery between January 2010 and December 2016. We identified 2,093 LT recipients with observation periods of 1 to 8 years.
RESULTS
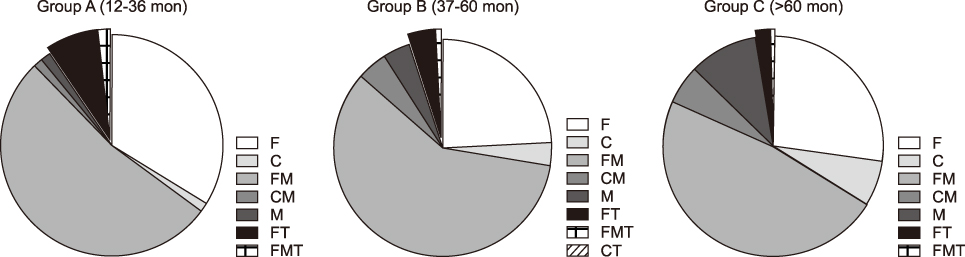
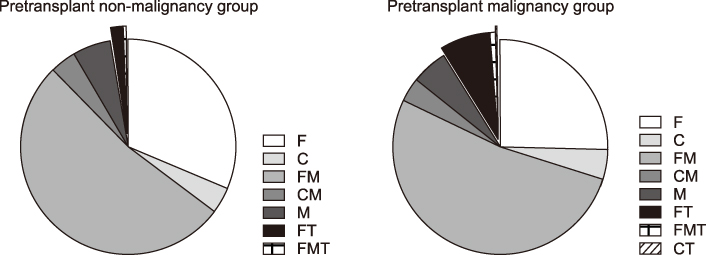
We divided the 2,093 recipients into three groups according to the posttransplant follow-up period as follows: group A (12-36 months; n=680), group B (37-60 months; n=560), and group C (>60 months; n=853). The individual IS agents were tacrolimus in 1,807 patients (86.3%), cyclosporine in 169 patients (8.1%), mycophenolate mofetil (MMF) in 1,310 patients (62.6%), and everolimus in 115 patients (5.5%). The most common IS regimens were tacrolimus-MMF combination and tacrolimus monotherapy, regardless of the posttransplant period. Patients with pretransplant malignancies were administered everolimus more frequently than those without pretransplant malignancies (P<0.001). In 102 patients with hepatocellular carcinoma recurrence or de novo malignancies, IS regimens included everolimus-tacrolimus in 41 patients (40.2%), tacrolimus-MMF in 27 patients (26.4%), tacrolimus in 20 patients (19.6%), MMF in 10 patients (9.8%), cyclosporine in three patients (2.9%), and cyclosporine-MMF in one patient (1.0%).
CONCLUSIONS
Administration of everolimus after LT has been gradually increasing with the expansion of indications in our institutional practice. Currently, the role of everolimus is minimal and not comparable to that of tacrolimus, but it has a unique position in the field of IS after LT.
MeSH Terms
Figure
Cited by 5 articles
-
Effect of everolimus rescue therapy for acute cellular rejection following pediatric living donor liver transplantation: Report of one case
Shin Hwang, Jung-Man Namgoong, Seak Hee Oh, Kyung Mo Kim, Chul-Soo Ahn, Hyunhee Kwon, Yu Jeong Cho, Yong Jae Kwon
Ann Hepatobiliary Pancreat Surg. 2020;24(2):216-220. doi: 10.14701/ahbps.2020.24.2.216.Whole liver deceased donor liver transplantation for pediatric recipients: single-center experience for 20 years
Jung-Man Namgoong, Shin Hwang, Dae-Yeon Kim, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Gil-Chun Park, Kyung Mo Kim, Seak Hee Oh
Korean J Transplant. 2020;34(4):249-256. doi: 10.4285/kjt.20.0036.Early use of everolimus improved renal function after adult deceased donor liver transplantation
Seohee Lee, Jong Man Kim, Sangjin Kim, Jinsoo Rhu, Gyu-Seong Choi, Jae-Won Joh
Korean J Transplant. 2021;35(1):8-14. doi: 10.4285/kjt.20.0043.Salvage living donor liver transplantation for post-resection recurrence of combined hepatocellular carcinoma-cholangiocarcinoma
Minjae Kim, Shin Hwang, Gi-Won Song, Chul-Soo Ahn, Deok-Bog Moon, Dong-Hwan Jung, Gil-Chun Park, Sung-Gyu Lee
Korean J Transplant. 2021;35(2):116-123. doi: 10.4285/kjt.20.0037.Twenty-year longitudinal follow-up after liver transplantation: a single-center experience with 251 consecutive patients
Minjae Kim, Shin Hwang, Chul-Soo Ahn, Deok-Bog Moon, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Gil-Chun Park, Ki-Hun Kim, Jung-Man Namgoong, Woo-Hyoung Kang, Young-In Yoon, Hwui-Dong Cho, Byeong-Gon Na, Sang Hoon Kim, Sung-Gyu Lee
Korean J Transplant. 2022;36(1):45-53. doi: 10.4285/kjt.21.0031.
Reference
-
1. Kang SH, Hwang S, Ha TY, Song GW, Jung DH, Kim KH, et al. Tailored long-term immunosuppressive regimen for adult liver transplant recipients with hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg. 2014; 18:48–51.
Article2. Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, Ha TY, et al. A clinical assessment of mycophenolate drug monitoring after liver transplantation. Clin Transplant. 2010; 24:E35–E42.
Article3. Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, et al. A cross-sectional analysis of long-term immunosuppressive regimens after liver transplantation at Asan Medical Center: increased preference for mycophenolate mofetil. Ann Hepatobiliary Pancreat Surg. 2018; 22:19–26.
Article4. Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012; 13:e11–e22.
Article5. Jung DH, Tak E, Hwang S, Song GW, Ahn CS, Kim KH, et al. Antitumor effect of sorafenib and mammalian target of rapamycin inhibitor in liver transplantation recipients with hepatocellular carcinoma recurrence. Liver Transpl. 2018; 24:932–945.
Article6. Fischer L, Saliba F, Kaiser GM, De Carlis L, Metselaar HJ, De Simone P, et al. Three-year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: follow-up results from a randomized, multicenter study. Transplantation. 2015; 99:1455–1462.
Article7. Alshahrani AA, Ha SM, Hwang S, Ahn CS, Kim KH, Moon DB, et al. Clinical features and surveillance of very late hepatocellular carcinoma recurrence after liver transplantation. Ann Transplant. 2018; 23:659–665.
Article8. Alshahrani AA, Hwang S, Song GW, Moon DB, Jung DH, Ahn CS, et al. Management of very late peritoneal metastasis of hepatocellular carcinoma 10 years after liver transplantation: lessons from two cases. Ann Hepatobiliary Pancreat Surg. 2018; 22:136–143.
Article9. Park YH, Hwang S, Song GW, Jung DH, Ahn CS, Kim KH, et al. Correlation between mycophenolic acid blood level and renal dysfunction in stable liver transplant recipients receiving mycophenolate monotherapy. Transplant Proc. 2014; 46:811–815.
Article10. Hwang S, Song GW, Jung DH, Park GC, Ahn CS, Moon DB, et al. Intra-individual variability of mycophenolic acid concentration according to renal function in liver transplant recipients receiving mycophenolate monotherapy. Ann Hepatobiliary Pancreat Surg. 2017; 21:11–16.
Article11. Yamanaka K, Petrulionis M, Lin S, Gao C, Galli U, Richter S, et al. Therapeutic potential and adverse events of everolimus for treatment of hepatocellular carcinoma: systematic review and meta-analysis. Cancer Med. 2013; 2:862–871.
Article12. Navarro-Villarán E, Tinoco J, Jiménez G, Pereira S, Wang J, Aliseda S, et al. Differential antitumoral properties and renal-associated tissue damage induced by tacrolimus and mammalian target of rapamycin inhibitors in hepatocarcinoma: in vitro and in vivo studies. PLoS One. 2016; 11:e0160979.
Article13. Zheng JF, Lu J, Wang XZ, Guo WH, Zhang JX. Comparative metabolomic profiling of hepatocellular carcinoma cells treated with sorafenib monotherapy vs. sorafenib-everolimus combination therapy. Med Sci Monit. 2015; 21:1781–1791.
Article14. Cholongitas Ε, Goulis I, Theocharidou E, Antoniadis N, Fouzas I, Giakoustidis D, et al. Everolimus-based immunosuppression in liver transplant recipients: a single-centre experience. Hepatol Int. 2014; 8:137–145.
Article15. Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014; 27:1039–1049.
Article16. Gomez-Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, et al. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012; 18:45–52.
Article17. De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012; 12:3008–3020.
Article18. Saliba F, De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, et al. Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 2013; 13:1734–1745.
Article19. Jiménez-Pérez M, González Grande R, Rando Muñoz FJ, de la Cruz Lombardo J, Muñoz Suárez MA, Fernández Aguilar JL, et al. Everolimus plus mycophenolate mofetil as initial immunosuppression in liver transplantation. Transplant Proc. 2015; 47:90–92.
Article20. Bilbao I, Dopazo C, Castells L, Lazaro J, Caralt M, Sapisochin G, et al. Immunosuppression based on everolimus in liver transplant recipients with severe early post-transplantation neurotoxicity. Transplant Proc. 2014; 46:3104–3107.
Article21. Beckebaum S, Cicinnati VR, Radtke A, Kabar I. Calcineurin inhibitors in liver transplantation: still champions or threatened by serious competitors. Liver Int. 2013; 33:656–665.
Article22. Herzer K, Strassburg CP, Braun F, Engelmann C, Guba M, Lehner F, et al. Selection and use of immunosuppressive therapies after liver transplantation: current German practice. Clin Transplant. 2016; 30:487–501.
Article23. Alegre C, Jiménez C, Manrique A, Abradelo M, Calvo J, Loinaz C, et al. Everolimus monotherapy or combined therapy in liver transplantation: indications and results. Transplant Proc. 2013; 45:1971–1974.
Article24. Saliba F, Dharancy S, Lorho R, Conti F, Radenne S, Neau-Cransac M, et al. Conversion to everolimus in maintenance liver transplant patients: a multicenter, retrospective analysis. Liver Transpl. 2011; 17:905–913.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A cross-sectional analysis of long-term immunosuppressive regimens after liver transplantation at Asan Medical Center: Increased preference for mycophenolate mofetil
- Tailored long-term immunosuppressive regimen for adult liver transplant recipients with hepatocellular carcinoma
- Current Status of Pediatric Liver Transplantation
- What's New in Transplantation Surgery and Medicine
- The immunosuppressive effects of liver regeneration factor after 30% and 100% liver transplantation in rat