A cross-sectional analysis of long-term immunosuppressive regimens after liver transplantation at Asan Medical Center: Increased preference for mycophenolate mofetil
- Affiliations
-
- 1Division of Hepatobiliary Surgery and Liver Transplantation, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. shwang@amc.seoul.kr
- KMID: 2409076
- DOI: http://doi.org/10.14701/ahbps.2018.22.1.19
Abstract
- BACKGROUNDS/AIMS
Long-term immunosuppression regimens after liver transplantation (LT) are rarely reported in detail. We aimed to provide information on actual long-term immunosuppression regimens through this cross-sectional study.
METHODS
Our institutional LT database was searched for adult patients who underwent primary LT operation from 2000 to 2016. We identified 3620 live recipients with actual information on immunosuppressive agent use for 1-17 years.
RESULTS
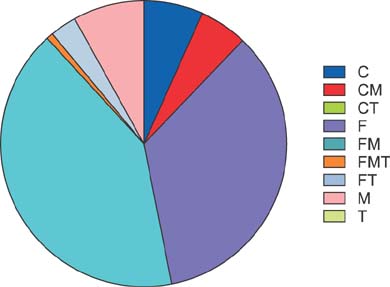
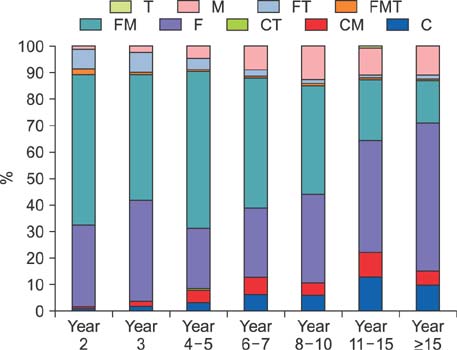
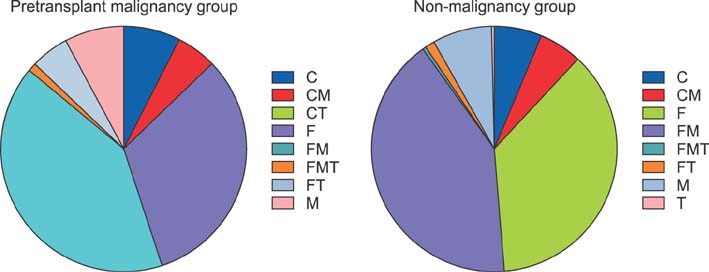
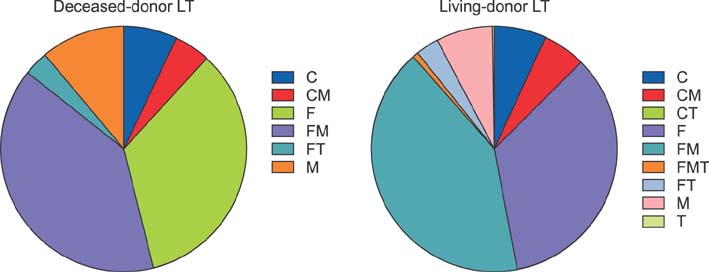
The study cohort was divided into 7 groups according to posttransplantation period. The immunosuppressive agents used at the cross-sectional review period were tacrolimus in 2884 (79.7%), cyclosporine in 445 (12.3%), mycophenolate mofetil in 2007 (55.4%), and everolimus in 138 (3.8%) recipients. There was no marked difference in immunosuppressive agent use according to pretransplantation liver malignancy or type of LT operation. Tacrolimus, cyclosporine, mycophenolate mofetil, and everolimus were used in 97.4%, 1.8%, 60.9%, and 9.2%, respectively, in the year 2 group; 94.1%, 3.9%, 51.6%, and 8.3%, respectively, in the year 3 group; 87.3%, 8.4%, 68.9%, and 4.8%, respectively, in the year 4-5 group; 78.2%, 12.9%, 64.6%, and 3.0%, respectively, in the year 6-7 group; 76.9%, 10.8%, 58.8%, and 2.4%, respectively, in the year 8-10 group; 66.7%, 22.4%, 43.4%, and 1.5%, respectively, in the year 11-15 group; and 73.8%, 15.4%, 32.9%, and 1.7%, respectively, in the year ≥15 group.
CONCLUSIONS
Tacrolimus and mycophenolate mofetil are the primary immunosuppressive agents after LT, and the indications for everolimus have started to increase at our institution. We believe our results will help establish tailored long-term immunosuppression regimens.
MeSH Terms
Figure
Cited by 4 articles
-
Cross-sectional analysis of immunosuppressive regimens focused on everolimus after liver transplantation in a Korean high-volume transplantation center
Sang-Hyun Kang, Shin Hwang, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Chul-Soo Ahn, Deok-Bog Moon, Ki-Hun Kim, Gil-Chun Park, Young-In Yoon, Yo-Han Park, Hui-Dong Cho, Jae-Hyun Kwon, Yong-Kyu Chung, Jin Uk Choi, Sung-Gyu Lee
Korean J Transplant. 2019;33(4):98-105. doi: 10.4285/jkstn.2019.33.4.98.Assessment of patient safety and the efficiency of facility utilization following simplified ultra-rapid intravenous infusion of hepatitis B immunoglobulin in a high-volume liver transplantation center
I-Ji Jeong, Shin Hwang, Dong-Hwan Jung, Gi-Won Song, Gil-Chun Park, Chul-Soo Ahn, Deok-Bog Moon, Ki-Hun Kim, Tae-Yong Ha, Hea-Seon Ha, Jung-Ja Hong, In-Ok Kim, Sung-Gyu Lee
Ann Hepatobiliary Pancreat Surg. 2019;23(2):128-132. doi: 10.14701/ahbps.2019.23.2.128.Pretransplant Hepatic Malignancy Increases Risk of De Novo Malignancy after Liver Transplantation
Gil-Chun Park, Shin Hwang, Chul-Soo Ahn, Ki-Hun Kim, Deok-Bog Moon, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Young-In Yoon, Hui-Dong Cho, Jae-Hyun Kwon, Yong-Kyu Chung, Sang-Hyun Kang, Jin-Uk Choi, I-Ji Jung, Sung-Gyu Lee
J Korean Med Sci. 2020;35(11):. doi: 10.3346/jkms.2020.35.e69.Twenty-year longitudinal follow-up after liver transplantation: a single-center experience with 251 consecutive patients
Minjae Kim, Shin Hwang, Chul-Soo Ahn, Deok-Bog Moon, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Gil-Chun Park, Ki-Hun Kim, Jung-Man Namgoong, Woo-Hyoung Kang, Young-In Yoon, Hwui-Dong Cho, Byeong-Gon Na, Sang Hoon Kim, Sung-Gyu Lee
Korean J Transplant. 2022;36(1):45-53. doi: 10.4285/kjt.21.0031.
Reference
-
1. Kang SH, Hwang S, Ha TY, Song GW, Jung DH, Kim KH, et al. Tailored long-term immunosuppressive regimen for adult liver transplant recipients with hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg. 2014; 18:48–51.
Article2. Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, Ha TY, et al. A clinical assessment of mycophenolate drug monitoring after liver transplantation. Clin Transplant. 2010; 24:E35–E42.
Article3. Park YH, Hwang S, Song GW, Jung DH, Ahn CS, Kim KH, et al. Correlation between mycophenolic acid blood level and renal dysfunction in stable liver transplant recipients receiving mycophenolate monotherapy. Transplant Proc. 2014; 46:811–815.
Article4. Hwang S, Song GW, Jung DH, Park GC, Ahn CS, Moon DB, et al. Intra-individual variability of mycophenolic acid concentration according to renal function in liver transplant recipients receiving mycophenolate monotherapy. Ann Hepatobiliary Pancreat Surg. 2017; 21:11–16.
Article5. Sugawara Y, Kaneko J, Makuuchi M. Cyclosporin a for treatment of hepatitis C virus after liver transplantation. Transplantation. 2006; 82:579–580.
Article6. Lionetti R, Calvaruso V, Piccolo P, Mancusi RL, Mazzarelli C, Fagiuoli S, et al. Sofosbuvir plus daclatasvir with or without ribavirin is safe and effective for post-transplant hepatitis C recurrence and severe fibrosis and cirrhosis: a prospective study. Clin Transplant. 2018; 32.
Article7. Dharancy S, Coilly A, Fougerou-Leurent C, Duvoux C, Kamar N, Leroy V, et al. Direct-acting antiviral agent-based regimen for HCV recurrence after combined liver-kidney transplantation: Results from the ANRS CO23 CUPILT study. Am J Transplant. 2017; 17:2869–2878.
Article8. Abaalkhail F, Elsiesy H, Elbeshbeshy H, Shawkat M, Yousif S, Ullah W, et al. Treatment of patients with hepatitis C virus infection with ledipasvir-sofosbuvir in the liver transplant setting. Transplantation. 2017; 101:2739–2745.
Article9. Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS Jr, Hassan MA, et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017; 66:1090–1101.
Article10. Huynh H, Ngo VC, Koong HN, Poon D, Choo SP, Thng CH, et al. Sorafenib and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Cell Mol Med. 2009; 13:2673–2683.
Article11. Yamanaka K, Petrulionis M, Lin S, Gao C, Galli U, Richter S, et al. Therapeutic potential and adverse events of everolimus for treatment of hepatocellular carcinoma - systematic review and meta-analysis. Cancer Med. 2013; 2:862–871.
Article12. Navarro-Villarán E, Tinoco J, Jiménez G, Pereira S, Wang J, Aliseda S, et al. Differential antitumoral properties and renal-associated tissue damage induced by tacrolimus and mammalian target of rapamycin inhibitors in hepatocarcinoma: in vitro and in vivo studies. PLoS One. 2016; 11:e0160979.
Article13. Zheng JF, Lu J, Wang XZ, Guo WH, Zhang JX. Comparative metabolomic profiling of hepatocellular carcinoma cells treated with sorafenib monotherapy vs. sorafenib-everolimus combination therapy. Med Sci Monit. 2015; 21:1781–1791.
Article14. Cholongitas E, Goulis I, Theocharidou E, Antoniadis N, Fouzas I, Giakoustidis D, et al. Everolimus-based immunosuppression in liver transplant recipients: a single-centre experience. Hepatol Int. 2014; 8:137–145.
Article15. Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014; 27:1039–1049.
Article16. Gomez-Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, et al. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012; 18:45–52.
Article17. Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012; 12:1855–1865.
Article18. Rodríguez-Perálvarez M, Germani G, Darius T, Lerut J, Tsochatzis E, Burroughs AK. Reducing early exposure to calcineurin inhibitors: the key factor for a successful renal sparing strategy. Am J Transplant. 2013; 13:239.
Article19. Jiménez-Pérez M, González Grande R, Rando Muñoz FJ, de la Cruz Lombardo J, Muñoz Suárez MA, Fernández Aguilar JL, et al. Everolimus plus mycophenolate mofetil as initial immunosuppression in liver transplantation. Transplant Proc. 2015; 47:90–92.
Article20. Bilbao I, Dopazo C, Castells L, Lazaro J, Caralt M, Sapisochin G, et al. Immunosuppression based on everolimus in liver transplant recipients with severe early post-transplantation neurotoxicity. Transplant Proc. 2014; 46:3104–3107.
Article21. Beckebaum S, Cicinnati VR, Radtke A, Kabar I. Calcineurin inhibitors in liver transplantation - still champions or threatened by serious competitors? Liver Int. 2013; 33:656–665.
Article22. Herzer K, Strassburg CP, Braun F, Engelmann C, Guba M, Lehner F, et al. Selection and use of immunosuppressive therapies after liver transplantation: current German practice. Clin Transplant. 2016; 30:487–501.
Article23. Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010; 51:1237–1243.
Article24. Namgoong JM, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, et al. A pilot study on the safety and efficacy of generic mycophenolate agent as conversion maintenance therapy in stable liver transplant recipients. Transplant Proc. 2013; 45:3035–3037.
Article25. Kim JM, Kwon CH, Yun IJ, Lee KW, Yu HC, Suh KS, et al. A multicenter experience with generic mycophenolate mofetil conversion in stable liver transplant recipients. Ann Surg Treat Res. 2014; 86:192–198.
Article26. Cassuto E, Pageaux GP, Cantarovich D, Rostaing L, Loupy A, Roche B, et al. Adherence to and acceptance of once-daily tacrolimus after kidney and liver transplant: results from OSIRIS, a French observational study. Transplantation. 2016; 100:2099–2106.27. Trunečka P. Once-daily tacrolimus in liver transplantation: a ‘me-too drug’, or a therapeutic advantage. Curr Opin Organ Transplant. 2017; 22:118–122.28. Ekong UD. The long-term liver graft and protocol biopsy: do we want to look? What will we find? Curr Opin Organ Transplant. 2011; 16:505–508.29. Hübscher SG. What is the long-term outcome of the liver allograft? J Hepatol. 2011; 55:702–717.
Article30. Mells G, Mann C, Hubscher S, Neuberger J. Late protocol liver biopsies in the liver allograft: a neglected investigation? Liver Transpl. 2009; 15:931–938.
Article31. Aguiar D, Martínez-Urbistondo D, D'Avola D, Iñarrairaegui M, Pardo F, Rotellar F, et al. Conversion from calcineurin inhibitor-based immunosuppression to mycophenolate mofetil in monotherapy reduces risk of de novo malignancies after liver transplantation. Ann Transplant. 2017; 22:141–147.
Article32. Koehl GE, Wagner F, Stoeltzing O, Lang SA, Steinbauer M, Schlitt HJ, et al. Mycophenolate mofetil inhibits tumor growth and angiogenesis in vitro but has variable antitumor effects in vivo, possibly related to bioavailability. Transplantation. 2007; 83:607–614.
Article33. Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, Graeb C, et al. The janus face of immunosuppression - de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney Int. 2007; 71:1271–1278.
Article34. Robson R, Cecka JM, Opelz G, Budde M, Sacks S. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant. 2005; 5:2954–2960.
Article35. Lake JR, David KM, Steffen BJ, Chu AH, Gordon RD, Wiesner RH. Addition of MMF to dual immunosuppression does not increase the risk of malignant short-term death after liver transplantation. Am J Transplant. 2005; 5:2961–2967.
Article36. O'Neill JO, Edwards LB, Taylor DO. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006; 25:1186–1191.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tailored long-term immunosuppressive regimen for adult liver transplant recipients with hepatocellular carcinoma
- Pretransplant mycophenolate mofetil reduces intrahepatic cholangiopathy related to laparoscopic donor hepatectomy in ABO-incompatible liver transplantation
- Cross-sectional analysis of immunosuppressive regimens focused on everolimus after liver transplantation in a Korean high-volume transplantation center
- The Efficacy and Outcome of Reduced Dose of Tacrolimus in Renal Transplantation
- History of organ transplantation and the development of key immunosuppressants