Chonnam Med J.
2020 Jan;56(1):6-11. 10.4068/cmj.2020.56.1.6.
Chimeric Antigen Receptor T Cell Therapy: A Novel Modality for Immune Modulation
- Affiliations
-
- 1Department of Pharmacology, Chonnam National University Medical School, Hwasun, Korea. eomgh@jnu.ac.kr
- KMID: 2468140
- DOI: http://doi.org/10.4068/cmj.2020.56.1.6
Abstract
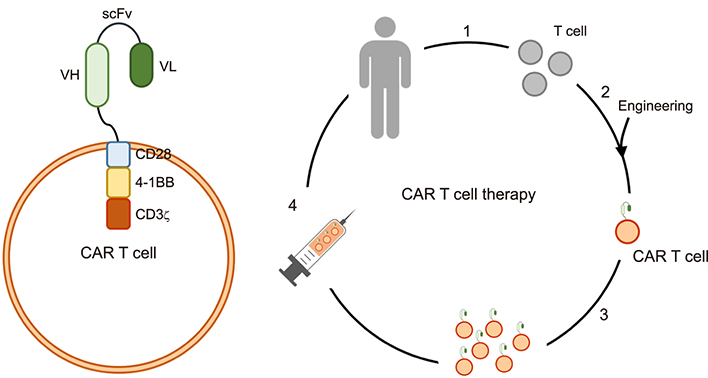
- Cancer remains a leading cause of death, despite multimodal treatment approaches. Even in patients with a healthy immune response, cancer cells can escape the immune system during tumorigenesis. Cancer cells incapacitate the normal cell-mediated immune system by expressing immune modulation ligands such as programmed death (PD) ligand 1, the B7 molecule, or secreting activators of immune modulators. Chimeric antigen receptor (CAR) T cells were originally designed to target cancer cells. Engineered approaches allow CAR T cells, which possess a simplified yet specific receptor, to be easily activated in limited situations. CAR T cell treatment is a derivative of the antigen-antibody reaction and can be applied to various diseases. In this review, the current successes of CAR T cells in cancer treatment and the therapeutic potential of CAR T cells are discussed.
Keyword
MeSH Terms
Figure
Reference
-
1. Rosso JD, Zeichner J, Alexis A, Cohen D, Berson D. Understanding the epidermal barrier in healthy and compromised skin: clinically relevant information for the dermatology practitioner: proceedings of an expert panel roundtable meeting. J Clin Aesthet Dermatol. 2016; 9:4 Suppl 1. S2–S8.2. Lawn SD, Butera ST, Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 2001; 14:753–777. table of contents.
Article3. Parkin J, Cohen B. An overview of the immune system. Lancet. 2001; 357:1777–1789.
Article4. Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006; 126:32–41.
Article5. Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009; 27:591–619.
Article6. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018; 24:541–550.
Article7. Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007; 446:153–158.
Article8. Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009; 6:519–527.
Article9. Wei J, Wang F, Kong LY, Xu S, Doucette T, Ferguson SD, et al. miR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma. Cancer Res. 2013; 73:3913–3926.
Article10. Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015; 21:687–692.
Article11. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015; 348:124–128.
Article12. Seliger B, Marincola FM, Ferrone S, Abken H. The complex role of B7 molecules in tumor immunology. Trends Mol Med. 2008; 14:550–559.
Article13. Chou FC, Chen HY, Kuo CC, Sytwu HK. Role of galectins in tumors and in clinical immunotherapy. Int J Mol Sci. 2018; 19:E430.
Article14. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017; 17:559–572.
Article15. Dennis KL, Blatner NR, Gounari F, Khazaie K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr Opin Oncol. 2013; 25:637–645.
Article16. Anderson KG, Stromnes IM, Greenberg PD. Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell. 2017; 31:311–325.
Article17. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016; 127:3321–3330.
Article18. Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev. 2015; 263:68–89.
Article19. Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol. 2018; 15:31–46.
Article20. Miliotou AN, Papadopoulou LC. CAR T-cell therapy: a new era in cancer immunotherapy. Curr Pharm Biotechnol. 2018; 19:5–18.
Article21. Singh S, Kumar NK, Dwiwedi P, Charan J, Kaur R, Sidhu P, et al. Monoclonal antibodies: a review. Curr Clin Pharmacol. 2018; 13:85–99.
Article22. Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008; 60:1421–1434.
Article23. Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009; 157:220–233.
Article24. Charrot S, Hallam S. CAR-T cells: future perspectives. Hemasphere. 2019; 3:e188.
Article25. Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016; 3:16011.
Article26. Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. 2017; 5:22.
Article27. Hindmarsh P, Leis J. Retroviral DNA integration. Microbiol Mol Biol Rev. 1999; 63:836–843.
Article28. Nowrouzi A, Glimm H, von Kalle C, Schmidt M. Retroviral vectors: post entry events and genomic alterations. Viruses. 2011; 3:429–455.
Article29. Sadelain M, Papapetrou EP, Bushman FD. Safe harbours for the integration of new DNA in the human genome. Nat Rev Cancer. 2011; 12:51–58.
Article30. Mollanoori H, Shahraki H, Rahmati Y, Teimourian S. CRISPR/Cas9 and CAR-T cell, collaboration of two revolutionary technologies in cancer immunotherapy, an instruction for successful cancer treatment. Hum Immunol. 2018; 79:876–882.
Article31. Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013; 10:267–276.
Article32. Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015; 125:4017–4023.
Article33. Ledford H. Engineered cell therapy for cancer gets thumbs up from FDA advisers. Nature. 2017; 547:270.
Article34. Axicabtagene ciloleucel (Yescarta) for B-cell lymphoma. Med Lett Drugs Ther. 2018; 60:e122–e123.35. Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013; 1:26–31.
Article36. Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017; 7:1404–1419.
Article37. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019; 25:625–638.
Article38. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N Engl J Med. 2018; 378:439–448.
Article39. Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. 2019; 10:2040620719841581.
Article40. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017; 377:2531–2544.
Article41. Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019; 573:430–433.
Article42. Rosenblum MD, Remedios KA, Abbas AK. Mechanisms of human autoimmunity. J Clin Invest. 2015; 125:2228–2233.
Article43. Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016; 353:179–184.
Article44. Salles G, Barrett M, Foà R, Maurer J, O'Brien S, Valente N, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017; 34:2232–2273.
Article45. Blick SK, Scott LJ. Cetuximab: a review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs. 2007; 67:2585–2607.46. Plosker GL, Keam SJ. Omalizumab: a review of its use in the treatment of allergic asthma. BioDrugs. 2008; 22:189–204.47. Salmikangas P, Kinsella N, Chamberlain P. Chimeric antigen receptor T-cells (CAR T-cells) for cancer immunotherapy - moving target for industry? Pharm Res. 2018; 35:152.
Article48. Fan MH, Zhu Q, Li HH, Ra HJ, Majumdar S, Gulick DL, et al. Fibroblast activation protein (FAP) accelerates collagen degradation and clearance from lungs in mice. J Biol Chem. 2016; 291:8070–8089.
Article49. Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011; 8:108–118.
Article50. Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016; 3:41–53.
Article51. Barratt SL, Creamer A, Hayton C, Chaudhuri N. Idiopathic pulmonary fibrosis (IPF): an overview. J Clin Med. 2018; 7:E201.
Article52. Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018; 378:1811–1823.
Article53. Hay AE, Cheung MC. CAR T-cells: costs, comparisons, and commentary. J Med Econ. 2019; 22:613–615.
Article54. Caruana I, Diaconu I, Dotti G. From monoclonal antibodies to chimeric antigen receptors for the treatment of human malignancies. Semin Oncol. 2014; 41:661–666.
Article55. Combs SE, Han G, Mani N, Beruti S, Nerenberg M, Rimm DL. Loss of antigenicity with tissue age in breast cancer. Lab Invest. 2016; 96:264–269.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Engineered T Cell Receptor for Cancer Immunotherapy
- Chimeric Antigen Receptor-T Cell Therapy
- Chimeric Antigen Receptor T-Cell Therapy for Diffuse Large B-Cell Lymphoma
- Current Challenges in Chimeric Antigen Receptor T-cell Therapy in Patients With B-cell Lymphoid Malignancies
- Management of adverse events in young adults and children with acute B-cell lymphoblastic leukemia receiving anti-CD19 chimeric antigen receptor (CAR) T-cell therapy