J Clin Neurol.
2020 Jan;16(1):53-59. 10.3988/jcn.2020.16.1.53.
Real-Life Effectiveness and Tolerability of Perampanel in Pediatric Patients Aged 4 Years or Older with Epilepsy: A Korean National Multicenter Study
- Affiliations
-
- 1Department of Pediatrics, School of Medicine, Kyungpook National University, Daegu, Korea. shkwon@knu.ac.kr
- 2Department of Pediatrics, Pusan National University Children's Hospital, Pusan National University School of Medicine, Yangsan, Korea.
- 3Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 4Department of Pediatrics, College of Medicine, Chungbuk National University, Cheongju, Korea.
- 5Department of Pediatrics, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 2467784
- DOI: http://doi.org/10.3988/jcn.2020.16.1.53
Abstract
- BACKGROUND AND PURPOSE
The US Food and Drug Administration approval for perampanel has only recently been expanded to patients as young as 4 years, and so there have been few real-life studies of the effects of perampanel in pediatric patients. The aim of this study was to determine the long-term efficacy, factors affecting treatment response, and tolerability of perampanel as an add-on therapy in pediatric patients aged 4 years or older with epilepsy.
METHODS
This multicenter retrospective observational study collected data from pediatric epilepsy centers of four Korean national universities. Changes in the seizure frequency from baseline, adverse events, and retention rates were obtained at 3, 6, and 12 months. Adverse events and discontinuation profiles were obtained to assess tolerability.
RESULTS
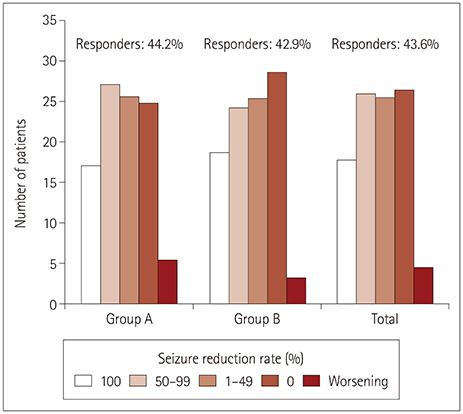
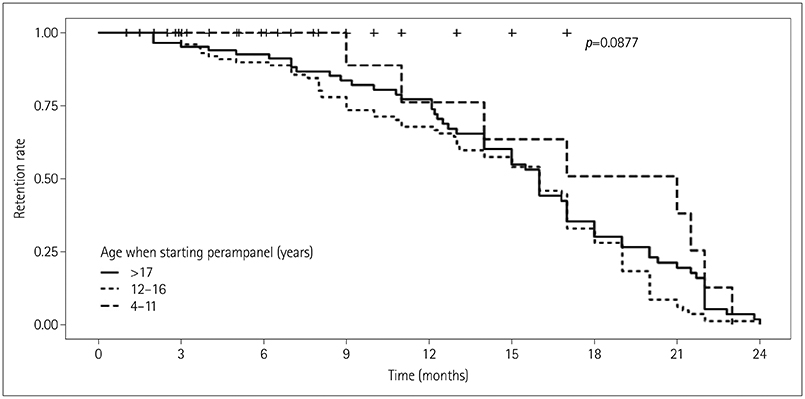
This study included 220 children and adolescents (117 males and 103 females) aged 4 to 20 years. The overall response rate was 43.6%, and the seizure-freedom rate was 17.7%. Factors affecting a good treatment response were the absence of intellectual disability, small number of concomitant antiepileptic drugs, and low baseline seizure frequency. Eighty-eight patients (40%) experienced adverse events, but they mostly were of mild severity and resolved after the dose reduction or discontinuation of perampanel. The retention rates at 3, 6, and 12 months were 85.0%, 71.8%, and 50.5%, respectively.
CONCLUSIONS
Adjunctive treatment with perampanel was efficacious and tolerated in pediatric patients aged 4 years or older with epilepsy. Early perampanel treatment may help to reduce the burden of their seizures and improve their quality of life.
MeSH Terms
Figure
Reference
-
1. Bhandage AK, Jin Z, Hellgren C, Korol SV, Nowak K, Williamsson L, et al. AMPA, NMDA and kainate glutamate receptor subunits are expressed in human peripheral blood mononuclear cells (PBMCs) where the expression of GluK4 is altered by pregnancy and GluN2D by depression in pregnant women. J Neuroimmunol. 2017; 305:51–58.
Article2. Kim HD, Chi CS, Desudchit T, Nikanorova M, Visudtibhan A, Nabangchang C, et al. Review of clinical studies of perampanel in adolescent patients. Brain Behav. 2016; 6:e00505.
Article3. Henley JM, Wilkinson KA. Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci. 2016; 17:337–350.
Article4. Rogawski MA. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr. 2011; 11:56–63.
Article5. Plosker GL. Perampanel: as adjunctive therapy in patients with partial-onset seizures. CNS Drugs. 2012; 26:1085–1096.6. Shih JJ, Tatum WO, Rudzinski LA. New drug classes for the treatment of partial onset epilepsy: focus on perampanel. Ther Clin Risk Manag. 2013; 9:285–293.
Article7. De Liso P, Moavero R, Coppola G, Curatolo P, Cusmai R, De Sarro G, et al. Current role of perampanel in pediatric epilepsy. Ital J Pediatr. 2017; 43:51.
Article8. Swiderska N, Tan HJ, Rajai A, Silwal A, Desurkar A, Martland T. Effectiveness and tolerability of perampanel in children, adolescents and young adults with refractory epilepsy: a UK national multicentre study. Seizure. 2017; 52:63–70.
Article9. Falco-Walter JJ, Scheffer IE, Fisher RS. The new definition and classification of seizures and epilepsy. Epilepsy Res. 2018; 139:73–79.
Article10. Biró A, Stephani U, Tarallo T, Bast T, Schlachter K, Fleger M, et al. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies: first experiences. Neuropediatrics. 2015; 46:110–116.
Article11. Datta AN, Xu Q, Sachedina S, Boelman C, Huh L, Connolly MB. Clinical experience with perampanel for refractory pediatric epilepsy in one Canadian center. J Child Neurol. 2017; 32:834–839.
Article12. De Liso P, Vigevano F, Specchio N, De Palma L, Bonanni P, Osanni E, et al. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies—An Italian observational multicenter study. Epilepsy Res. 2016; 127:93–100.
Article13. Heyman E, Lahat E, Levin N, Epstein O, Lazinger M, Berkovitch M, et al. Tolerability and efficacy of perampanel in children with refractory epilepsy. Dev Med Child Neurol. 2017; 59:441–444.
Article14. Lagae L, Villanueva V, Meador KJ, Bagul M, Laurenza A, Kumar D, et al. Adjunctive perampanel in adolescents with inadequately controlled partial-onset seizures: a randomized study evaluating behavior, efficacy, and safety. Epilepsia. 2016; 57:1120–1129.
Article15. Gidal BE, Laurenza A, Hussein Z, Yang H, Fain R, Edelstein J, et al. Perampanel efficacy and tolerability with enzyme-inducing AEDs in patients with epilepsy. Neurology. 2015; 84:1972–1980.
Article16. Rudzinski LA, Vélez-Ruiz NJ, Gedzelman ER, Mauricio EA, Shih JJ, Karakis I. New antiepileptic drugs: focus on ezogabine, clobazam, and perampanel. J Investig Med. 2016; 64:1087–1101.
Article17. Gidal BE, Ferry J, Majid O, Hussein Z. Concentration-effect relationships with perampanel in patients with pharmacoresistant partial-onset seizures. Epilepsia. 2013; 54:1490–1497.
Article18. Kwan P, Brodie MJ, Laurenza A, FitzGibbon H, Gidal BE. Analysis of pooled phase III trials of adjunctive perampanel for epilepsy: impact of mechanism of action and pharmacokinetics on clinical outcomes. Epilepsy Res. 2015; 117:117–124.
Article19. Tsai JJ, Wu T, Leung H, Desudchit T, Tiamkao S, Lim KS, et al. Perampanel, an AMPA receptor antagonist: from clinical research to practice in clinical settings. Acta Neurol Scand. 2018; 137:378–391.
Article20. Coyle H, Clough P, Cooper P, Mohanraj R. Clinical experience with perampanel: focus on psychiatric adverse effects. Epilepsy Behav. 2014; 41:193–196.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Tolerability of Low-Dose Perampanel in Patients with Childhood-Onset Intractable Epilepsy
- New antiepileptic drugs: usage, adverse effects, and interactions
- Adverse Events During Perampanel Adjunctive Therapy in Intractable Epilepsy
- Real-Life Efficacy and Tolerability of Lacosamide in Pediatric Patients Aged 4 Years or Older with Drug-Resistant Epilepsy
- Five-Year Retention of Perampanel and Polytherapy Patterns: 328 Patients From a Single Center in South Korea