J Clin Neurol.
2018 Jul;14(3):296-302. 10.3988/jcn.2018.14.3.296.
Adverse Events During Perampanel Adjunctive Therapy in Intractable Epilepsy
- Affiliations
-
- 1Divison of Pediatric Neurology, Department of Pediatrics, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea. hdkimmd@yuhs.ac
- 2Department of Pediatrics, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2415044
- DOI: http://doi.org/10.3988/jcn.2018.14.3.296
Abstract
- BACKGROUND AND PURPOSE
Perampanel is the first α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA)-receptor antagonist developed to treat epilepsy. The effects of either rapid or slow dose titration on adverse events remain to be elucidated.
METHODS
Eighty-five patients received perampanel between March 2016 and August 2016. Patients were divided into two groups according to their dosing schedule: rapid dose titration (2-mg increments at intervals of 1 to 2 weeks) and slow dose titration (2-mg increments at intervals of at least 3 weeks). Seizure frequency and adverse events were analyzed over 3 months.
RESULTS
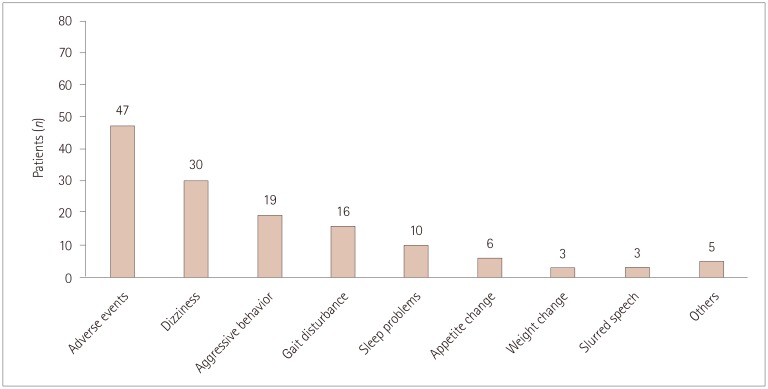
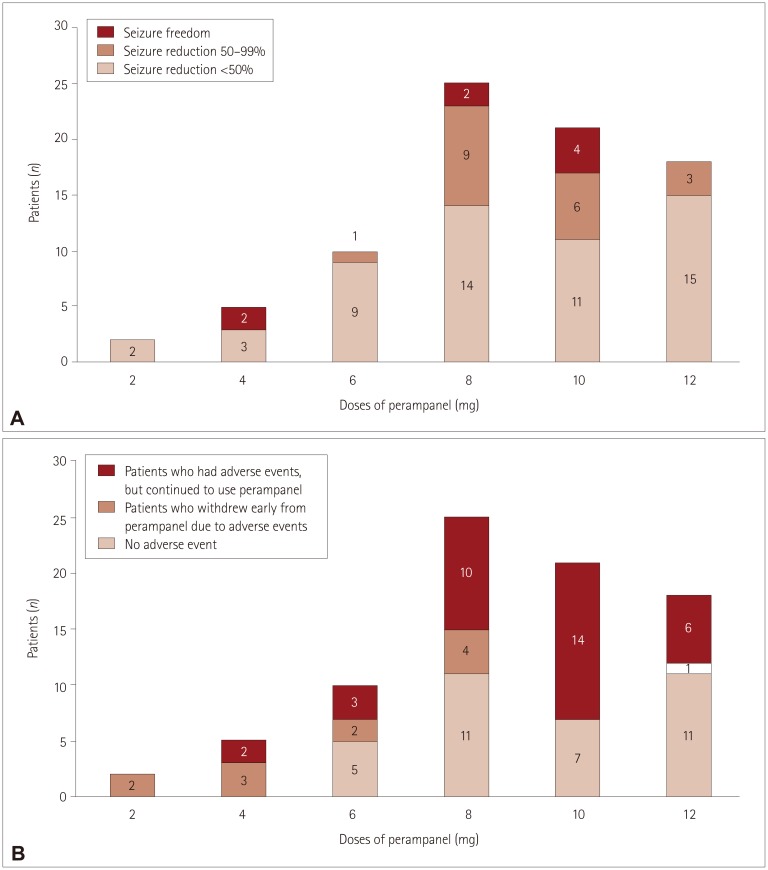
Adverse events were reported by 47 (58%) of the 81 patients analyzed, with 12 (15%) patients discontinuing perampanel due to adverse events. Common adverse events included dizziness (n=30, 37%), aggressive mood and behavior (n=19, 24%), gait disturbance (n=16, 20%), and sleep problems (n=10, 12.4%). The overall adverse events were similar in the slow-titration group (38 of 61 patients) and the rapid-titration group (8 of 20 patients, p=0.081). However, none of the 20 patients in the slow-titration group experienced gait disturbance, compared with 16 of the 61 patients in the rapid-titration group (p=0.009), while appetite change was experienced by 4 patients in the slow-titration group but only 1 in the rapid-titration group (p=0.003). No relationship was noted between adverse events and the maximum dose of perampanel (p=0.116). Sex differences were observed, with the response to perampanel being better and the rate of adverse events being higher in females (p=0.015 and p=0.046, respectively).
CONCLUSIONS
Slow titration of perampanel may reduce perampanel-related adverse events.
Keyword
MeSH Terms
Figure
Reference
-
1. Rogawski MA. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr. 2011; 11:56–63. PMID: 21686307.
Article2. French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012; 79:589–596. PMID: 22843280.
Article3. French JA, Krauss GL, Steinhoff BJ, Squillacote D, Yang H, Kumar D, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013; 54:117–125. PMID: 22905857.
Article4. Krauss GL, Serratosa JM, Villanueva V, Endziniene M, Hong Z, French J, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012; 78:1408–1415. PMID: 22517103.
Article5. De Liso P, Vigevano F, Specchio N, De Palma L, Bonanni P, Osanni E, et al. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies: an Italian observational multicenter study. Epilepsy Res. 2016; 127:93–100. PMID: 27568598.6. Steinhoff BJ, Hamer H, Trinka E, Schulze-Bonhage A, Bien C, Mayer T, et al. A multicenter survey of clinical experiences with perampanel in real life in Germany and Austria. Epilepsy Res. 2014; 108:986–988. PMID: 24721197.
Article7. Steinhoff BJ, Bacher M, Bast T, Kornmeier R, Kurth C, Scholly J, et al. First clinical experiences with perampanel--the Kork experience in 74 patients. Epilepsia. 2014; 55(Suppl 1):16–18.
Article8. Shah E, Reuber M, Goulding P, Flynn C, Delanty N, Kemp S. Clinical experience with adjunctive perampanel in adult patients with uncontrolled epilepsy: a UK and Ireland multicentre study. Seizure. 2016; 34:1–5. PMID: 26615577.
Article9. Heyman E, Lahat E, Levin N, Epstein O, Lazinger M, Berkovitch M, et al. Tolerability and efficacy of perampanel in children with refractory epilepsy. Dev Med Child Neurol. 2017; 59:441–444. PMID: 27935018.
Article10. Biró A, Stephani U, Tarallo T, Bast T, Schlachter K, Fleger M, et al. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies: first experiences. Neuropediatrics. 2015; 46:110–116. PMID: 25730374.
Article11. Patsalos PN. The clinical pharmacology profile of the new antiepileptic drug perampanel: a novel noncompetitive AMPA receptor antagonist. Epilepsia. 2015; 56:12–27. PMID: 25495693.
Article12. Fisher RS. The new classification of seizures by the international league against epilepsy 2017. Curr Neurol Neurosci Rep. 2017; 17:48. PMID: 28425015.
Article13. Schalock RL, Borthwick-Duffy SA, Buntinx WHE, Coulter DL, Craig EM. Intellectual disability: definition, classification, and systems of supports. 11th ed. Washington, D.C.: American Association on Intellectual and Developmental Disabilities;2010.14. Trinka E, Steinhoff BJ, Nikanorova M, Brodie MJ. Perampanel for focal epilepsy: insights from early clinical experience. Acta Neurol Scand. 2016; 133:160–172. PMID: 26506904.
Article15. Gidal BE, Ferry J, Majid O, Hussein Z. Concentration-effect relationships with perampanel in patients with pharmacoresistant partial-onset seizures. Epilepsia. 2013; 54:1490–1497. PMID: 23772853.
Article16. Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007; 55:81–95. PMID: 17129734.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Tolerability of Low-Dose Perampanel in Patients with Childhood-Onset Intractable Epilepsy
- Real-Life Effectiveness and Tolerability of Perampanel in Pediatric Patients Aged 4 Years or Older with Epilepsy: A Korean National Multicenter Study
- New antiepileptic drugs: usage, adverse effects, and interactions
- Efficacy of levetiracetam in refractory childhood epilepsy
- A meta-analysis: efficacy and safety of anti-epileptic drugs prescribed in Korea as monotherapy and adjunctive treatment for patients with focal epilepsy