J Clin Neurol.
2019 Oct;15(4):488-495. 10.3988/jcn.2019.15.4.488.
Combined Assessment of Serum Alpha-Synuclein and Rab35 is a Better Biomarker for Parkinson's Disease
- Affiliations
-
- 1Neuroscience Research Center, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan. ccchei@cgmh.org.tw
- 2Healthy Aging Research Center, Chang Gung University, Taoyuan, Taiwan.
- 3Department of Physiology and Pharmacology, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
- 4Division of Movement Disorders, Department of Neurology, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan.
- 5College of Medicine, Chang Gung University, Taoyuan, Taiwan.
- 6Department of Neurology, Taipei Medical University Hospital, Taipei, Taiwan.
- 7Institute for Medical Informatics, Biometrics and Epidemiology, Ludwig-Maximilians-Universität, München, Germany.
- 8Institute of Cognitive Neuroscience, National Central University, Taoyuan, Taiwan.
- 9Department of Sports Medicine, Landseed Hospital, Taoyuan, Taiwan.
- 10Graduate Institute of Biomedical Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
- 11Department of Psychiatry, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan.
- 12Department of Nursing, Chang Gung University of Science and Technology, Taoyuan, Taiwan.
- 13Department of Medical Biotechnology and Laboratory Science, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
- KMID: 2467758
- DOI: http://doi.org/10.3988/jcn.2019.15.4.488
Abstract
- BACKGROUND AND PURPOSE
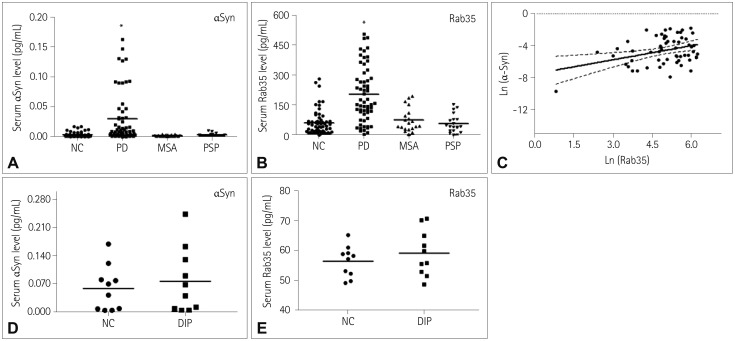
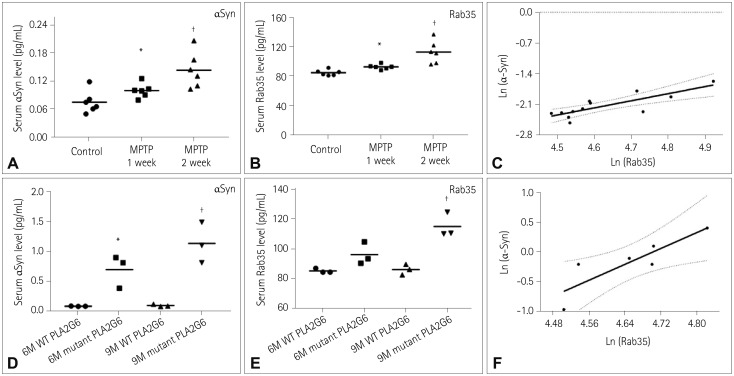
It is essential to develop a reliable predictive serum biomarker for Parkinson's disease (PD). The accumulation of alpha-synuclein (αSyn) and up-regulated expression of Rab35 participate in the etiology of PD. The purpose of this investigation was to determine whether the combined assessment of serum αSyn and Rab35 is a useful predictive biomarker for PD.
METHODS
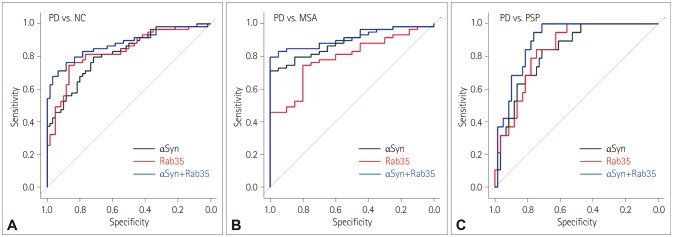
Serum levels of αSyn or Rab35 were determined in serum samples from 59 sporadic PD patients, 19 progressive supranuclear palsy (PSP) patients, 20 multiple system atrophy (MSA) patients, and 60 normal controls (NC). Receiver operating characteristics (ROC) curves were calculated to determine the diagnostic accuracy of αSyn or/and Rab35 in discriminating PD patients from NC or atypical parkinsonian patients.
RESULTS
The levels of αSyn and Rab35 were increased in PD patients. The serum level of Rab35 was positively correlated with that of αSyn in PD patients. Compared to analyzing αSyn or Rab35 alone, the combined analysis of αSyn and Rab35 produced a larger area under the ROC curve and performed better in discriminating PD patients from NC, MSA patients, or PSP patients. When age was dichotomized at 55, 60, 65, or 70 years, the combined assessment of αSyn and Rab35 for classifying PD was better in the group below the cutoff age than in the group above the cutoff age.
CONCLUSIONS
Combined assessment of serum αSyn and Rab35 is a better biomarker for discriminating PD patients from NC or atypical parkinsonian patients, and is a useful predictive biomarker for younger sporadic PD patients.
Keyword
MeSH Terms
Figure
Reference
-
1. De Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006; 5:525–535. PMID: 16713924.
Article2. Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008; 79:368–376. PMID: 18344392.
Article3. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997; 388:839–840. PMID: 9278044.4. Burré J. The synaptic function of α-synuclein. J Parkinsons Dis. 2015; 5:699–713. PMID: 26407041.
Article5. Karpinar DP, Balija MB, Kügler S, Opazo F, Rezaei-Ghaleh N, Wender N, et al. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson's disease models. EMBO J. 2009; 28:3256–3268. PMID: 19745811.6. El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, et al. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 2006; 20:419–425. PMID: 16507759.7. Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011; 10:230–240. PMID: 21317042.
Article8. Park MJ, Cheon SM, Bae HR, Kim SH, Kim JW. Elevated levels of α-synuclein oligomer in the cerebrospinal fluid of drug-naïve patients with Parkinson's disease. J Clin Neurol. 2011; 7:215–222. PMID: 22259618.
Article9. Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013; 14:38–48. PMID: 23254192.
Article10. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009; 10:513–525. PMID: 19603039.
Article11. Dalfó E, Gómez-Isla T, Rosa JL, Nieto Bodelón M, Cuadrado Tejedor M, Barrachina M, et al. Abnormal alpha-synuclein interactions with Rab proteins in alpha-synuclein A30P transgenic mice. J Neuropathol Exp Neurol. 2004; 63:302–313. PMID: 15099020.12. Gao Y, Wilson GR, Stephenson SEM, Bozaoglu K, Farrer MJ, Lockhart PJ. The emerging role of Rab GTPases in the pathogenesis of Parkinson's disease. Mov Disord. 2018; 33:196–207. PMID: 29315801.
Article13. Wilson GR, Sim JC, McLean C, Giannandrea M, Galea CA, Riseley JR, et al. Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with α-synuclein pathology. Am J Hum Genet. 2014; 95:729–735. PMID: 25434005.
Article14. Atashrazm F, Hammond D, Perera G, Bolliger MF, Matar E, Halliday GM, et al. LRRK2-mediated Rab10 phosphorylation in immune cells from Parkinson's disease patients. Mov Disord. 2019; 34:406–415. PMID: 30597610.
Article15. Mir R, Tonelli F, Lis P, Macartney T, Polinski NK, Martinez TN, et al. The Parkinson's disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem J. 2018; 475:1861–1883. PMID: 29743203.
Article16. Kiral FR, Kohrs FE, Jin EJ, Hiesinger PR. Rab GTPases and membrane trafficking in neurodegeneration. Curr Biol. 2018; 28:R471–R486. PMID: 29689231.17. Lai YC, Kondapalli C, Lehneck R, Procter JB, Dill BD, Woodroof HI, et al. Phosphoproteomic screening identifies Rab GTPases as novel downstream targets of PINK1. EMBO J. 2015; 34:2840–2861. PMID: 26471730.18. Yamano K, Wang C, Sarraf SA, Münch C, Kikuchi R, Noda NN, et al. Endosomal Rab cycles regulate Parkin-mediated mitophagy. Elife. 2018; 7:e31326. PMID: 29360040.
Article19. Rivero-Ríos P, Romo-Lozano M, Madero-Pérez J, Thomas AP, Biosa A, Greggio E, et al. The G2019S variant of leucine-rich repeat kinase 2 (LRRK2) alters endolysosomal trafficking by impairing the function of the GTPase RAB8A. J Biol Chem. 2019; 294:4738–4758. PMID: 30709905.
Article20. Bae EJ, Kim DK, Kim C, Mante M, Adame A, Rockenstein E, et al. LRRK2 kinase regulates α-synuclein propagation via RAB35 phosphorylation. Nat Commun. 2018; 9:3465. PMID: 30150626.
Article21. Madero-Pérez J, Fdez E, Fernández B, Lara Ordóñez AJ, Blanca Ramírez M, Gómez-Suaga P, et al. Parkinson disease-associated mutations in LRRK2 cause centrosomal defects via Rab8a phosphorylation. Mol Neurodegener. 2018; 13:3. PMID: 29357897.
Article22. Fujimoto T, Kuwahara T, Eguchi T, Sakurai M, Komori T, Iwatsubo T. Parkinson's disease-associated mutant LRRK2 phosphorylates Rab7L1 and modifies trans-Golgi morphology. Biochem Biophys Res Commun. 2018; 495:1708–1715. PMID: 29223392.
Article23. Purlyte E, Dhekne HS, Sarhan AR, Gomez R, Lis P, Wightman M, et al. Rab29 activation of the Parkinson's disease-associated LRRK2 kinase. EMBO J. 2018; 37:1–18. PMID: 29212815.
Article24. Jeong GR, Jang EH, Bae JR, Jun S, Kang HC, Park CH, et al. Dysregulated phosphorylation of Rab GTPases by LRRK2 induces neurode-generation. Mol Neurodegener. 2018; 13:8. PMID: 29439717.
Article25. Chutna O, Gonçalves S, Villar-Piqué A, Guerreiro P, Marijanovic Z, Mendes T, et al. The small GTPase Rab11 co-localizes with α-synuclein in intracellular inclusions and modulates its aggregation, secretion and toxicity. Hum Mol Genet. 2014; 23:6732–6745. PMID: 25092884.
Article26. Chiu CC, Yeh TH, Lai SC, Weng YH, Huang YC, Cheng YC, et al. Increased Rab35 expression is a potential biomarker and implicated in the pathogenesis of Parkinson's disease. Oncotarget. 2016; 7:54215–54227. PMID: 27509057.
Article27. Pagan FL. Improving outcomes through early diagnosis of Parkinson's disease. Am J Manag Care. 2012; 18:S176–S182. PMID: 23039866.28. Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson's disease. Lancet Neurol. 2006; 5:75–86. PMID: 16361025.
Article29. Yang SY, Chiu MJ, Lin CH, Horng HE, Yang CC, Chieh JJ, et al. Development of an ultra-high sensitive immunoassay with plasma biomarker for differentiating Parkinson disease dementia from Parkinson disease using antibody functionalized magnetic nanoparticles. J Nanobiotechnology. 2016; 14:41. PMID: 27278241.
Article30. Shin HW, Chung SJ. Drug-induced parkinsonism. J Clin Neurol. 2012; 8:15–21. PMID: 22523509.
Article31. Chiu CC, Lu CS, Weng YH, Chen YL, Huang YZ, Chen RS, et al. PARK14 (D331Y) PLA2G6 causes early-onset degeneration of substantia nigra dopaminergic neurons by inducing mitochondrial dysfunction, ER stress, mitophagy impairment and transcriptional dysregulation in a knockin mouse model. Mol Neurobiol. 2019; 56:3835–3853. PMID: 30088174.
Article32. Chiu CC, Yeh TH, Lai SC, Wu-Chou YH, Chen CH, Mochly-Rosen D, et al. Neuroprotective effects of aldehyde dehydrogenase 2 activation in rotenone-induced cellular and animal models of parkinsonism. Exp Neurol. 2015; 263:244–253. PMID: 25263579.
Article33. Schapira AH. Science, medicine, and the future: Parkinson's disease. BMJ. 1999; 318:311–314. PMID: 9924061.
Article34. Schlesinger I, Schlesinger N. Uric acid in Parkinson's disease. Mov Disord. 2008; 23:1653–1657. PMID: 18618666.
Article35. Brodacki B, Staszewski J, Toczyłowska B, Kozłowska E, Drela N, Chalimoniuk M, et al. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci Lett. 2008; 441:158–162. PMID: 18582534.36. Scalzo P, Kümmer A, Cardoso F, Teixeira AL. Serum levels of interleukin-6 are elevated in patients with Parkinson's disease and correlate with physical performance. Neurosci Lett. 2010; 468:56–58. PMID: 19857551.
Article37. Godau J, Herfurth M, Kattner B, Gasser T, Berg D. Increased serum insulin-like growth factor 1 in early idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010; 81:536–538. PMID: 20176597.
Article38. Cui SS, Du JJ, Liu SH, Meng J, Lin YQ, Li G, et al. Serum soluble lymphocyte activation gene-3 as a diagnostic biomarker in Parkinson's disease: a pilot multicenter study. Mov Disord. 2019; 34:138–141. PMID: 30485547.
Article39. Marques TM, Van Rumund A, Oeckl P, Kuiperij HB, Esselink RAJ, Bloem BR, et al. Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology. 2019; 92:e1479–e1486. PMID: 30814322.
Article40. Bagheri V, Khorramdelazad H, Hassanshahi G, Moghadam-Ahmadi A, Vakilian A. CXCL12 and CXCR4 in the peripheral blood of patients with Parkinson's disease. Neuroimmunomodulation. 2018; 25:201–205. PMID: 30428473.
Article41. Niimi Y, Ito S, Mizutani Y, Murate K, Shima S, Ueda A, et al. Altered regulation of serum lysosomal acid hydrolase activities in Parkinson's disease: a potential peripheral biomarker. Parkinsonism Relat Disord. 2019; 61:132–137. PMID: 30415794.
Article42. Pavlos NJ, Grønborg M, Riedel D, Chua JJ, Boyken J, Kloepper TH, et al. Quantitative analysis of synaptic vesicle Rabs uncovers distinct yet overlapping roles for Rab3a and Rab27b in Ca2+-triggered exocytosis. J Neurosci. 2010; 30:13441–13453. PMID: 20926670.
Article43. Gerges NZ, Backos DS, Esteban JA. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J Biol Chem. 2004; 279:43870–43878. PMID: 15297461.
Article44. Mignogna ML, Giannandrea M, Gurgone A, Fanelli F, Raimondi F, Mapelli L, et al. The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition. Nat Commun. 2015; 6:6504. PMID: 25784538.
Article45. Mori Y, Fukuda M, Henley JM. Small GTPase Rab17 regulates the surface expression of kainate receptors but not α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in hippocampal neurons via dendritic trafficking of Syntaxin-4 protein. J Biol Chem. 2014; 289:20773–20787. PMID: 24895134.
Article46. Shin N, Jeong H, Kwon J, Heo HY, Kwon JJ, Yun HJ, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008; 314:2055–2065. PMID: 18445495.
Article47. Shi MM, Shi CH, Xu YM. Rab GTPases: the key players in the molecular pathway of Parkinson's disease. Front Cell Neurosci. 2017; 11:81. PMID: 28400718.
Article48. Williams SM, Schulz P, Sierks MR. Oligomeric α-synuclein and β- amyloid variants as potential biomarkers for Parkinson's and Alzheimer's diseases. Eur J Neurosci. 2016; 43:3–16. PMID: 26332448.49. Brigo F, Erro R, Marangi A, Bhatia K, Tinazzi M. Differentiating drug-induced parkinsonism from Parkinson's disease: an update on non-motor symptoms and investigations. Parkinsonism Relat Disord. 2014; 20:808–814. PMID: 24935237.
Article50. Wu Y, Le W, Jankovic J. Preclinical biomarkers of Parkinson disease. Arch Neurol. 2011; 68:22–30. PMID: 21220674.
Article51. Sherer TB. Biomarkers for Parkinson's disease. Sci Transl Med. 2011; 3:79ps14.
Article52. Haehner A, Boesveldt S, Berendse HW, Mackay-Sim A, Fleischmann J, Silburn PA, et al. Prevalence of smell loss in Parkinson's disease--a multicenter study. Parkinsonism Relat Disord. 2009; 15:490–494. PMID: 19138875.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Structure, Distribution, and Genetic Profile of α-Synuclein and Their Potential Clinical Application in Parkinson's Disease
- Alpha-Synuclein Function and Dysfunction on Cellular Membranes
- Accumulation of Alpha-synuclein Causes Colonic Dysmotility Independently of Enteric Nervous Damage in the Early Stage of Parkinson's Disease (Neurogastroenterol Motil 2012;24:e425-e436)
- Alpha-Synuclein Expression in Patients with Parkinson's Disease: A Clinician's Perspective
- ATP13A2 and Alpha-synuclein: a Metal Taste in Autophagy