Korean J Radiol.
2019 Jul;20(7):1003-1018. 10.3348/kjr.2018.0611.
Response Assessment with MRI after Chemoradiotherapy in Rectal Cancer: Current Evidences
- Affiliations
-
- 1Department of Radiology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. jslim1@yuhs.ac
- 2Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2467019
- DOI: http://doi.org/10.3348/kjr.2018.0611
Abstract
- Baseline magnetic resonance imaging (MRI) has become the primary staging modality for surgical plans and stratification of patient populations for more efficient neoadjuvant treatment. Patients who exhibit a complete response to chemoradiotherapy (CRT) may achieve excellent local tumor control and better quality of life with organ-preserving treatments such as local excision or even watch-and-wait management. Therefore, the evaluation of tumor response is a key factor for determining the appropriate treatment following CRT. Although post-CRT MRI is generally accepted as the first-choice method for evaluating treatment response after CRT, its application in the clinical decision process is not fully validated. In this review, we will discuss various oncologic treatment options from radical surgical technique to organ-preservation strategies for achieving better cancer control and improved quality of life following CRT. In addition, the current status of post-CRT MRI in restaging rectal cancer as well as the main imaging features that should be evaluated for treatment planning will also be described for the tailored treatment.
Keyword
MeSH Terms
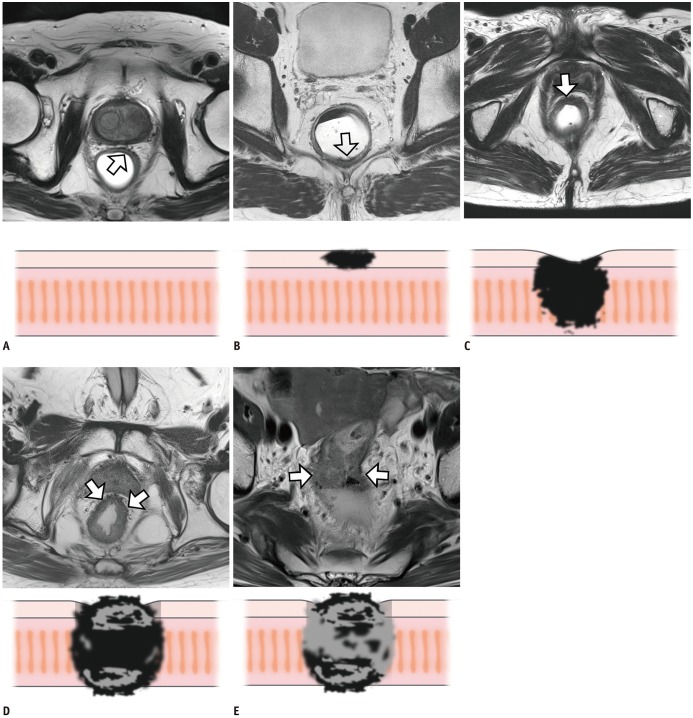
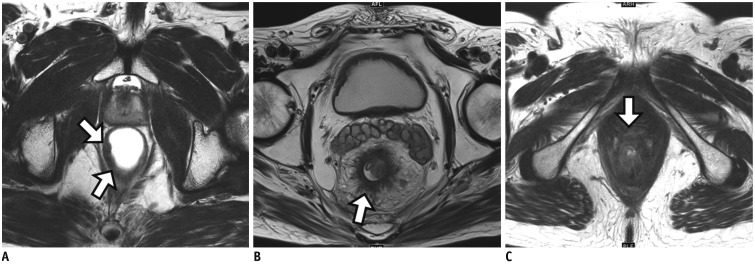
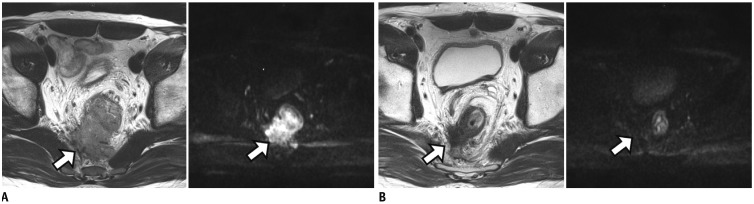
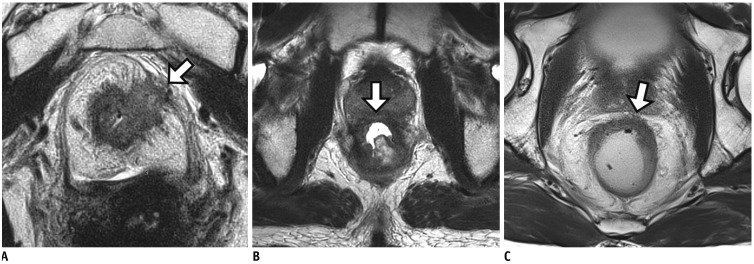
Figure
Reference
-
1. KSAR Study Group for Rectal Cancer. Essential items for structured reporting of rectal cancer MRI: 2016 consensus recommendation from the Korean Society of Abdominal Radiology. Korean J Radiol. 2017; 18:132–151. PMID: 28096724.2. Costa-Silva L, Brown G. Magnetic resonance imaging of rectal cancer. Magn Reson Imaging Clin N Am. 2013; 21:385–408. PMID: 23642559.
Article3. García-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003; 46:298–304. PMID: 12626903.
Article4. Glynne-Jones R. Rectal cancer--the times they are a-changing. Lancet Oncol. 2012; 13:651–653. PMID: 22627103.
Article5. Tepper JE, O'Neil BH. Minimizing therapy and maximizing outcomes in rectal cancer. J Clin Oncol. 2011; 29:4604–4606. PMID: 22067395.
Article6. Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986; 1:1479–1482. PMID: 2425199.
Article7. Kim NK, Sugihara K, Liang JT. Surgical treatment of colorectal cancer: Asian perspectives on optimization and standardization. Singapore: Springer Singapore;2018.8. Kuvshinoff B, Maghfoor I, Miedema B, Bryer M, Westgate S, Wilkes J, et al. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: are < or = 1 cm distal margins sufficient? Ann Surg Oncol. 2001; 8:163–169. PMID: 11258782.9. Battersby NJ, How P, Moran B, Stelzner S, West NP, Branagan G, et al. MERCURY II Study Group. Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: the MERCURY II study. Ann Surg. 2016; 263:751–760. PMID: 25822672.10. Shihab OC, Heald RJ, Rullier E, Brown G, Holm T, Quirke P, et al. Defining the surgical planes on MRI improves surgery for cancer of the low rectum. Lancet Oncol. 2009; 10:1207–1211. PMID: 19959077.
Article11. Nagtegaal ID, van de Velde CJ, Marijnen CA, van Krieken JH, Quirke P. Dutch Colorectal Cancer Group. Pathology Review Committee. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005; 23:9257–9264. PMID: 16361623.
Article12. Yu HC, Peng H, He XS, Zhao RS. Comparison of short- and long-term outcomes after extralevator abdominoperineal excision and standard abdominoperineal excision for rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2014; 29:183–191. PMID: 24271080.
Article13. Yamada K, Ishizawa T, Niwa K, Chuman Y, Akiba S, Aikou T. Patterns of pelvic invasion are prognostic in the treatment of locally recurrent rectal cancer. Br J Surg. 2001; 88:988–993. PMID: 11442533.
Article14. Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015; 33:1797–1808. PMID: 25918296.
Article15. Lambregts DM, Rao SX, Sassen S, Martens MH, Heijnen LA, Buijsen J, et al. MRI and diffusion-weighted MRI volumetry for identification of complete tumor responders after preoperative chemoradiotherapy in patients with rectal cancer: a Bi-institutional validation study. Ann Surg. 2015; 262:1034–1039. PMID: 25211270.16. Vliegen RF, Beets GL, Lammering G, Dresen RC, Rutten HJ, Kessels AG, et al. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology. 2008; 246:454–462. PMID: 18227541.
Article17. Cummings BJ, Rider WD, Harwood AR, Keane TJ, Thomas GM. Radical external beam radiation therapy for adenocarcinoma of the rectum. Dis Colon Rectum. 1983; 26:30–36. PMID: 6822158.
Article18. Goodman KA. Timing is everything: what is the optimal duration after chemoradiation for surgery for rectal cancer? J Clin Oncol. 2016; 34:3724–3728. PMID: 27601550.
Article19. Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol. 2016; 34:3773–3780. PMID: 27432930.
Article20. Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018; 28:1465–1475. PMID: 29043428.
Article21. Patel UB, Brown G, Rutten H, West N, Sebag-Montefiore D, Glynne-Jones R, et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2012; 19:2842–2852. PMID: 22526897.
Article22. Bhoday J, Smith F, Siddiqui MR, Balyasnikova S, Swift RI, Perez R, et al. Magnetic resonance tumor regression grade and residual mucosal abnormality as predictors for pathological complete response in rectal cancer postneoadjuvant chemoradiotherapy. Dis Colon Rectum. 2016; 59:925–933. PMID: 27602923.
Article23. Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008; 191:1827–1835. PMID: 19020255.
Article24. Patel UB, Blomqvist LK, Taylor F, George C, Guthrie A, Bees N, et al. MRI after treatment of locally advanced rectal cancer: how to report tumor response--the MERCURY experience. AJR Am J Roentgenol. 2012; 199:W486–W495. PMID: 22997398.
Article25. Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011; 29:3753–3760. PMID: 21876084.
Article26. Jang JK, Lee JL, Park SH, Park HJ, Park IJ, Kim JH, et al. Magnetic resonance tumour regression grade and pathological correlates in patients with rectal cancer. Br J Surg. 2018; 105:1671–1679. PMID: 29893988.
Article27. Battersby NJ, Dattani M, Rao S, Cunningham D, Tait D, Adams R, et al. A rectal cancer feasibility study with an embedded phase III trial design assessing magnetic resonance tumour regression grade (mrTRG) as a novel biomarker to stratify management by good and poor response to chemoradiotherapy (TRIGGER): study protocol for a randomised controlled trial. Trials. 2017; 18:394. PMID: 28851403.
Article28. van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. 2013; 269:101–112. PMID: 23801777.
Article29. Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008; 26:303–312. PMID: 18182672.
Article30. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012; 30:1926–1933. PMID: 22529255.
Article31. Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994; 344:707–711. PMID: 7915774.
Article32. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006; 333:779. PMID: 16984925.33. Kulkarni T, Gollins S, Maw A, Hobson P, Byrne R, Widdowson D. Magnetic resonance imaging in rectal cancer downstaged using neoadjuvant chemoradiation: accuracy of prediction of tumour stage and circumferential resection margin status. Colorectal Dis. 2008; 10:479–489. PMID: 18318754.
Article34. Kim KH, Park MJ, Lim JS, Kim NK, Min BS, Ahn JB, et al. Circumferential resection margin positivity after preoperative chemoradiotherapy based on magnetic resonance imaging for locally advanced rectal cancer: implication of boost radiotherapy to the involved mesorectal fascia. Jpn J Clin Oncol. 2016; 46:316–322. PMID: 26802164.
Article35. Kim SH, Lee JM, Park HS, Eun HW, Han JK, Choi BI. Accuracy of MRI for predicting the circumferential resection margin, mesorectal fascia invasion, and tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging. 2009; 29:1093–1101. PMID: 19388124.
Article36. Park MJ, Kim SH, Lee SJ, Jang KM, Rhim H. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy. Radiology. 2011; 260:771–780. PMID: 21846762.
Article37. Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003; 227:371–377. PMID: 12732695.
Article38. Perez RO, Pereira DD, Proscurshim I, Gama-Rodrigues J, Rawet V, São Julião GP, et al. Lymph node size in rectal cancer following neoadjuvant chemoradiation--can we rely on radiologic nodal staging after chemoradiation? Dis Colon Rectum. 2009; 52:1278–1284. PMID: 19571705.
Article39. Lahaye MJ, Beets GL, Engelen SM, Kessels AG, de Bruïne AP, Kwee HW, et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part II. What are the criteria to predict involved lymph nodes? Radiology. 2009; 252:81–89. PMID: 19403848.
Article40. Kusters M, Marijnen CA, van de Velde CJ, Rutten HJ, Lahaye MJ, Kim JH, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010; 36:470–476. PMID: 20096534.
Article41. Lahaye MJ, Engelen SM, Kessels AG, de Bruïne AP, von Meyenfeldt MF, van Engelshoven JM, et al. USPIO-enhanced MR imaging for nodal staging in patients with primary rectal cancer: predictive criteria. Radiology. 2008; 246:804–811. PMID: 18195379.
Article42. Engelen SM, Beets-Tan RG, Lahaye MJ, Lammering G, Jansen RL, van Dam RM, et al. MRI after chemoradiotherapy of rectal cancer: a useful tool to select patients for local excision. Dis Colon Rectum. 2010; 53:979–986. PMID: 20551748.
Article43. Koh DM, Chau I, Tait D, Wotherspoon A, Cunningham D, Brown G. Evaluating mesorectal lymph nodes in rectal cancer before and after neoadjuvant chemoradiation using thin-section T2-weighted magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2008; 71:456–461. PMID: 18164860.
Article44. Prall F, Wöhlke M, Klautke G, Schiffmann L, Fietkau R, Barten M. Tumour regression and mesorectal lymph node changes after intensified neoadjuvant chemoradiation for carcinoma of the rectum. APMIS. 2006; 114:201–210. PMID: 16643187.
Article45. Akiyoshi T, Ueno M, Matsueda K, Konishi T, Fujimoto Y, Nagayama S, et al. Selective lateral pelvic lymph node dissection in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy based on pretreatment imaging. Ann Surg Oncol. 2014; 21:189–196. PMID: 23963871.
Article46. Oh HK, Kang SB, Lee SM, Lee SY, Ihn MH, Kim DW, et al. Neoadjuvant chemoradiotherapy affects the indications for lateral pelvic node dissection in mid/low rectal cancer with clinically suspected lateral node involvement: a multicenter retrospective cohort study. Ann Surg Oncol. 2014; 21:2280–2287. PMID: 24604580.
Article47. Chand M, Evans J, Swift RI, Tekkis PP, West NP, Stamp G, et al. The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann Surg. 2015; 261:473–479. PMID: 25243543.
Article48. Lee ES, Kim MJ, Park SC, Hur BY, Hyun JH, Chang HJ, et al. Magnetic resonance imaging-detected extramural venous invasion in rectal cancer before and after preoperative chemoradiotherapy: diagnostic performance and prognostic significance. Eur Radiol. 2018; 28:496–505. PMID: 28786006.
Article49. Chand M, Swift RI, Tekkis PP, Chau I, Brown G. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer. 2014; 110:19–25. PMID: 24300971.
Article50. Ale Ali H, Kirsch R, Razaz S, Jhaveri A, Thipphavong S, Kennedy ED, et al. Extramural venous invasion in rectal cancer: overview of imaging, histopathology, and clinical implications. Abdom Radiol (NY). 2019; 44:1–10. PMID: 29967984.
Article51. Liang P, Nakada I, Hong JW, Tabuchi T, Motohashi G, Takemura A, et al. Prognostic significance of immunohistochemically detected blood and lymphatic vessel invasion in colorectal carcinoma: its impact on prognosis. Ann Surg Oncol. 2007; 14:470–477. PMID: 17103258.
Article52. Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999; 17:2396–2402. PMID: 10561302.
Article53. Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010; 11:835–844. PMID: 20692872.
Article54. Borschitz T, Wachtlin D, Möhler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol. 2008; 15:712–720. PMID: 18163173.
Article55. Park IJ, Yu CS. Current issues in locally advanced colorectal cancer treated by preoperative chemoradiotherapy. World J Gastroenterol. 2014; 20:2023–2029. PMID: 24587677.
Article56. Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011; 29:4633–4640. PMID: 22067400.
Article57. Maas M, Lambregts DM, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JW, et al. Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Ann Surg Oncol. 2015; 22:3873–3880. PMID: 26198074.
Article58. Barbaro B, Vitale R, Leccisotti L, Vecchio FM, Santoro L, Valentini V, et al. Restaging locally advanced rectal cancer with MR imaging after chemoradiation therapy. Radiographics. 2010; 30:699–716. PMID: 20462989.
Article59. van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, et al. IWWD Consortium. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018; 391:2537–2545. PMID: 29976470.60. Park SH, Lim JS, Lee J, Kim HY, Koom WS, Hur H, et al. Rectal mucinous adenocarcinoma: MR imaging assessment of response to concurrent chemotherapy and radiation therapy-A hypothesis-generating study. Radiology. 2017; 285:124–133. PMID: 28520513.
Article61. Sclafani F, Brown G, Cunningham D, Wotherspoon A, Mendes LST, Balyasnikova S, et al. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br J Cancer. 2017; 117:1478–1485. PMID: 28934761.
Article62. Kim S, Han K, Seo N, Kim HJ, Kim MJ, Koom WS, et al. T2-weighted signal intensity-selected volumetry for prediction of pathological complete response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2018; 28:5231–5240. PMID: 29858637.
Article63. Kluza E, Rozeboom ED, Maas M, Martens M, Lambregts DM, Slenter J, et al. T2 weighted signal intensity evolution may predict pathological complete response after treatment for rectal cancer. Eur Radiol. 2013; 23:253–261. PMID: 22777621.
Article64. Kim SH, Lee JM, Hong SH, Kim GH, Lee JY, Han JK, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009; 253:116–125. PMID: 19789256.
Article65. Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M, et al. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol. 2011; 18:2224–2231. PMID: 21347783.
Article66. Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011; 260:734–743. PMID: 21673229.
Article67. Gerard JP, Romestaing P, Chapet O. Radiotherapy alone in the curative treatment of rectal carcinoma. Lancet Oncol. 2003; 4:158–166. PMID: 12623361.
Article68. Habr-Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, Kiss D, et al. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006; 10:1319–1328. PMID: 17175450.69. Duldulao MP, Lee W, Streja L, Chu P, Li W, Chen Z, et al. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum. 2013; 56:142–149. PMID: 23303141.
Article70. Paty PB, Nash GM, Baron P, Zakowski M, Minsky BD, Blumberg D, et al. Long-term results of local excision for rectal cancer. Ann Surg. 2002; 236:522–529. discussion 529–530. PMID: 12368681.
Article71. Kim JG, Song KD, Kim SH, Kim HC, Huh JW. Diagnostic performance of MRI for prediction of candidates for local excision of rectal cancer (ypT0-1N0) after neoadjuvant chemoradiation therapy. J Magn Reson Imaging. 2016; 44:471–477. PMID: 26800999.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interpretation of Rectal MRI after Neoadjuvant Treatment in Patients with Rectal Cancer
- Imaging Diagnosis of Locally Advanced Rectal Cancer: Tumor Staging before and after Preoperative Chemoradiotherapy
- Combination Assessment of Clinical Complete Response of Patients With Rectal Cancer Following Chemoradiotherapy With Endoscopy and Magnetic Resonance Imaging
- Radiologic Report for Magnetic Resonance Imaging of Rectal Cancer before Treatment
- Surgical issues in locally advanced rectal cancer treated by preoperative chemoradiotherapy