Yonsei Med J.
2016 Jan;57(1):238-246. 10.3349/ymj.2016.57.1.238.
Effect of Pneumoperitoneum on Oxidative Stress and Inflammation via the Arginase Pathway in Rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Severance Hospital, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea. yjoh@yuhs.ac
- 2Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2466376
- DOI: http://doi.org/10.3349/ymj.2016.57.1.238
Abstract
- PURPOSE
Oxidative stress during CO2 pneumoperitoneum is reported to be associated with decreased bioactivity of nitric oxide (NO). However, the changes in endothelial nitric oxide synthase (eNOS), inducible nitric oxide synthase (iNOS), and arginase during CO2 pneumoperitoneum have not been elucidated.
MATERIALS AND METHODS
Thirty male Sprague-Dawley rats were randomized into three groups. After anesthesia induction, the abdominal cavities of the rats of groups intra-abdominal pressure (IAP)-10 and IAP-20 were insufflated with CO2 at pressures of 10 mm Hg and 20 mm Hg, respectively, for 2 hours. The rats of group IAP-0 were not insufflated. After deflation, plasma NO was measured, while protein expression levels and activity of eNOS, iNOS, arginase (Arg) I, and Arg II were analyzed with aorta and lung tissue samples.
RESULTS
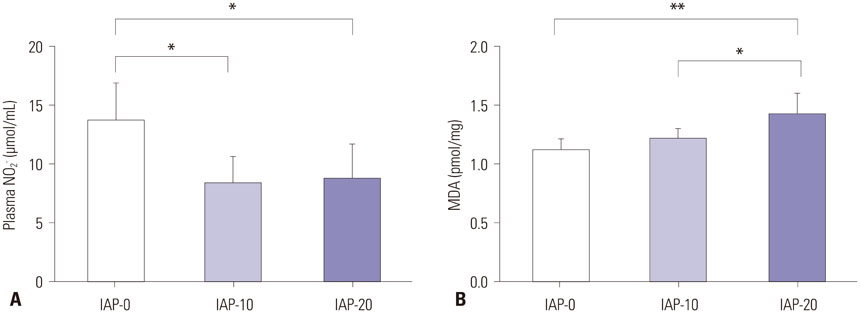
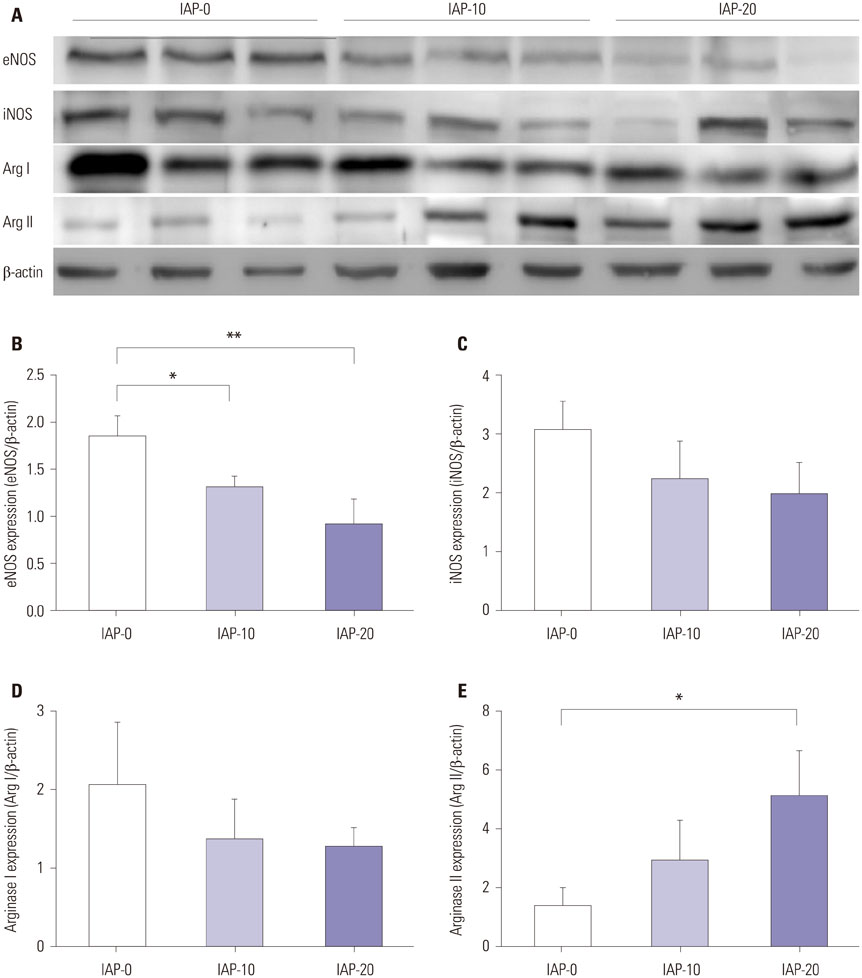
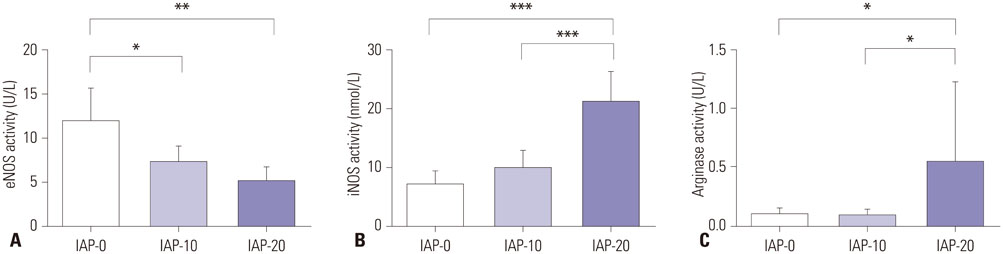
Plasma nitrite concentration and eNOS expression were significantly suppressed in groups IAP-10 and IAP-20 compared to IAP-0. While expression of iNOS and Arg I were comparable between the three groups, Arg II expression was significantly greater in group IAP-20 than in group IAP-0. Activity of eNOS was significantly lower in groups IAP-10 and IAP-20 than in group IAP-0, while iNOS activity was significantly greater in group IAP-20 than in groups IAP-0 and IAP-10. Arginase activity was significantly greater in group IAP-20 than in groups IAP-0 and IAP-10.
CONCLUSION
The activity of eNOS decreases during CO2 pneumoperitoneum, while iNOS activity is significantly increased, a change that contributes to increased oxidative stress and inflammation. Moreover, arginase expression and activity is increased during CO2 pneumoperitoneum, which seems to act inversely to the NO system.
MeSH Terms
-
Animals
Aorta/*physiology
Arginase/*antagonists & inhibitors
Enzyme Inhibitors/administration & dosage/pharmacology
Inflammation/etiology/*prevention & control
Injections, Subcutaneous
Lung Injury/etiology/prevention & control
Male
Nitric Oxide/metabolism
Nitric Oxide Synthase Type II/*metabolism
Nitric Oxide Synthase Type III/*metabolism
Oxidative Stress/*drug effects
Pneumoperitoneum/*complications/drug therapy
Rats
Rats, Sprague-Dawley
Arginase
Enzyme Inhibitors
Nitric Oxide
Nitric Oxide Synthase Type II
Nitric Oxide Synthase Type III
Figure
Reference
-
1. Sammour T, Mittal A, Loveday BP, Kahokehr A, Phillips AR, Windsor JA, et al. Systematic review of oxidative stress associated with pneumoperitoneum. Br J Surg. 2009; 96:836–850.
Article2. Abassi Z, Bishara B, Karram T, Khatib S, Winaver J, Hoffman A. Adverse effects of pneumoperitoneum on renal function: involvement of the endothelin and nitric oxide systems. Am J Physiol Regul Integr Comp Physiol. 2008; 294:R842–R850.
Article3. Ishizaki Y, Bandai Y, Shimomura K, Abe H, Ohtomo Y, Idezuki Y. Changes in splanchnic blood flow and cardiovascular effects following peritoneal insufflation of carbon dioxide. Surg Endosc. 1993; 7:420–423.
Article4. Nickkholgh A, Barro-Bejarano M, Liang R, Zorn M, Mehrabi A, Gebhard MM, et al. Signs of reperfusion injury following CO2 pneumoperitoneum: an in vivo microscopy study. Surg Endosc. 2008; 22:122–128.
Article5. Demyttenaere S, Feldman LS, Fried GM. Effect of pneumoperitoneum on renal perfusion and function: a systematic review. Surg Endosc. 2007; 21:152–160.
Article6. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012; 33:829–837.
Article7. Ali NA, Eubanks WS, Stamler JS, Gow AJ, Lagoo-Deenadayalan SA, Villegas L, et al. A method to attenuate pneumoperitoneum-induced reductions in splanchnic blood flow. Ann Surg. 2005; 241:256–261.
Article8. Shimazutsu K, Uemura K, Auten KM, Baldwin MF, Belknap SW, La Banca F, et al. Inclusion of a nitric oxide congener in the insufflation gas repletes S-nitrosohemoglobin and stabilizes physiologic status during prolonged carbon dioxide pneumoperitoneum. Clin Transl Sci. 2009; 2:405–412.
Article9. Bishara B, Karram T, Khatib S, Ramadan R, Schwartz H, Hoffman A, et al. Impact of pneumoperitoneum on renal perfusion and excretory function: beneficial effects of nitroglycerine. Surg Endosc. 2009; 23:568–576.
Article10. Bishara B, Abu-Saleh N, Awad H, Goltsman I, Ramadan R, Khamaysi I, et al. Pneumoperitoneum aggravates renal function in cases of decompensated but not compensated experimental congestive heart failure: role of nitric oxide. J Urol. 2011; 186:310–317.
Article11. Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007; 34:906–911.
Article12. Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003; 108:2000–2006.
Article13. Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol (1985). 2009; 107:1249–1257.
Article14. Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, et al. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res. 2007; 101:692–702.
Article15. Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, et al. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008; 102:923–932.16. Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006; 113:1708–1714.17. Kristof AS, Goldberg P, Laubach V, Hussain SN. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1998; 158:1883–1889.
Article18. Bishara B, Ramadan R, Karram T, Awad H, Abu-Saleh N, Winaver J, et al. Nitric oxide synthase inhibition aggravates the adverse renal effects of high but not low intraabdominal pressure. Surg Endosc. 2010; 24:826–833.
Article19. Ozmen MM, Zulfikaroglu B, Col C, Cinel I, Isman FK, Cinel L, et al. Effect of increased abdominal pressure on cytokines (IL1 beta, IL6, TNFalpha), C-reactive protein (CRP), free radicals (NO, MDA), and histology. Surg Laparosc Endosc Percutan Tech. 2009; 19:142–147.
Article20. Romeo C, Impellizzeri P, Antonuccio P, Turiaco N, Cifalá S, Gentile C, et al. Peritoneal macrophage activity after laparoscopy or laparotomy. J Pediatr Surg. 2003; 38:97–101.
Article21. Hajri A, Mutter D, Wack S, Bastien C, Gury JF, Marescaux J, et al. Dual effect of laparoscopy on cell-mediated immunity. Eur Surg Res. 2000; 32:261–266.
Article22. Morris SM Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol. 2009; 157:922–930.
Article23. Luiking YC, Ten Have GA, Wolfe RR, Deutz NE. Arginine de novo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab. 2012; 303:E1177–E1189.
Article24. Pross M, Schulz HU, Flechsig A, Manger T, Halangk W, Augustin W, et al. Oxidative stress in lung tissue induced by CO(2) pneumoperitoneum in the rat. Surg Endosc. 2000; 14:1180–1184.
Article25. Sare M, Hamamci D, Yilmaz I, Birincioglu M, Mentes BB, Ozmen M, et al. Effects of carbon dioxide pneumoperitoneum on free radical formation in lung and liver tissues. Surg Endosc. 2002; 16:188–192.
Article26. Karapolat S, Gezer S, Yildirim U, Dumlu T, Karapolat B, Ozaydin I, et al. Prevention of pulmonary complications of pneumoperitoneum in rats. J Cardiothorac Surg. 2011; 6:14.
Article27. Lin CC, Hsieh NK, Liou HL, Chen HI. Niacinamide mitigated the acute lung injury induced by phorbol myristate acetate in isolated rat's lungs. J Biomed Sci. 2012; 19:27.
Article28. Ni YF, Kuai JK, Lu ZF, Yang GD, Fu HY, Wang J, et al. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression. J Surg Res. 2011; 165:e29–e35.
Article29. Mealy K, Gallagher H, Barry M, Lennon F, Traynor O, Hyland J. Physiological and metabolic responses to open and laparoscopic cholecystectomy. Br J Surg. 1992; 79:1061–1064.
Article30. Cho JM, LaPorta AJ, Clark JR, Schofield MJ, Hammond SL, Mallory PL 2nd. Response of serum cytokines in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 1994; 8:1380–1383.
Article31. Glaser F, Sannwald GA, Buhr HJ, Kuntz C, Mayer H, Klee F, et al. General stress response to conventional and laparoscopic cholecystectomy. Ann Surg. 1995; 221:372–380.
Article32. Pernow J, Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal. Cardiovasc Res. 2013; 98:334–343.
Article33. Jeyabalan G, Klune JR, Nakao A, Martik N, Wu G, Tsung A, et al. Arginase blockade protects against hepatic damage in warm ischemia-reperfusion. Nitric Oxide. 2008; 19:29–35.
Article34. Gonon AT, Jung C, Katz A, Westerblad H, Shemyakin A, Sjöquist PO, et al. Local arginase inhibition during early reperfusion mediates cardioprotection via increased nitric oxide production. PLoS One. 2012; 7:e42038.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Oxidative Stress in the Pathogenesis of Asthma
- The role of oxidative stress and hypoxia in renal disease
- Effects of S-allylcysteine on Oxidative Stress in Streptozotocin-Induced Diabetic Rats
- The Role of Reactive Oxygen Species, Inflammation, and Endoplasmic Reticulum Stress Response in the Finasteride Protective Effect against Benign Prostate Hyperplasia
- Cerulein Pancreatitis: Oxidative Stress, Inflammation, and Apoptosis