Yonsei Med J.
2020 Jan;61(1):48-55. 10.3349/ymj.2020.61.1.48.
Comparison of Real-World Outcomes of Infliximab versus Adalimumab in Biologic-Naïve Korean Patients with Ulcerative Colitis: A Population-Based Study
- Affiliations
-
- 1Biostatistics Collaboration Unit, Department of Biomedical Systems Informatics, Yonsei University College of Medicine, Seoul, Korea.
- 2Division of Gastroenterology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Internal Medicine and Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea. GENIUSHEE@yuhs.ac
- 4Department of Biostatistics, Graduate School of Public Health, Yonsei University, Seoul, Korea. soheepark@yuhs.ac
- KMID: 2466335
- DOI: http://doi.org/10.3349/ymj.2020.61.1.48
Abstract
- PURPOSE
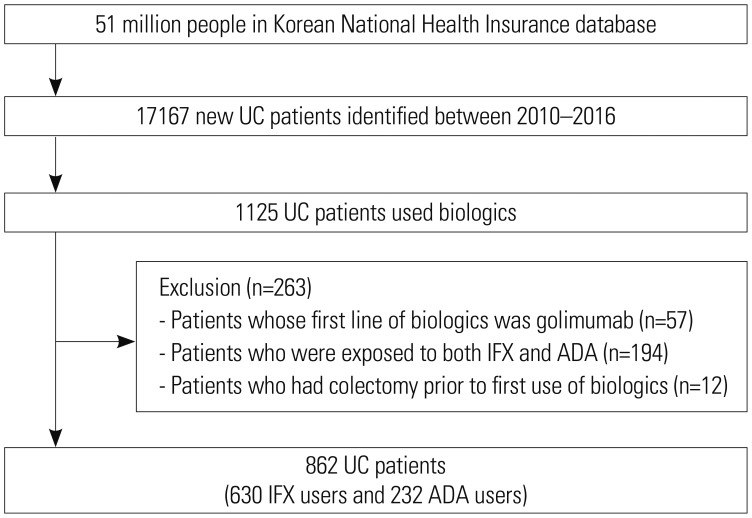
Data on the comparative effectiveness of infliximab (IFX) or adalimumab (ADA) in patients with ulcerative colitis (UC) are extremely limited, especially in the Asian population. We compared clinically important outcomes [colectomy, UC-related emergency room (ER) visits, UC-related hospitalizations, and need for corticosteroids] for these two biologics in biologic-naïve Korean patients with UC.
MATERIALS AND METHODS
Using National Health Insurance claims, we collected data on patients who were diagnosed with UC and exposed to IFX or ADA between 2010 and 2016.
RESULTS
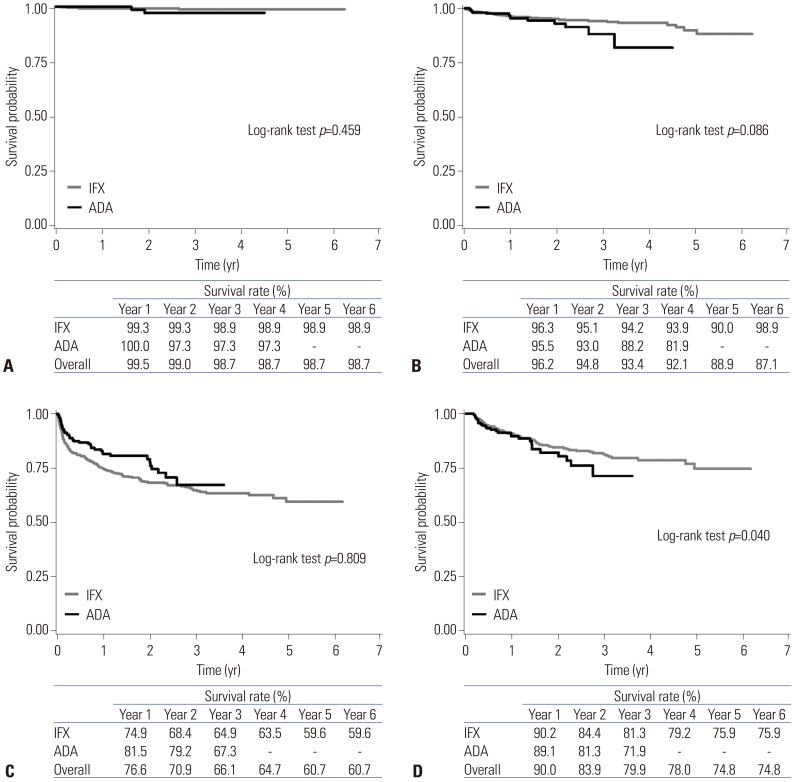
A total of 862 new users of biologics were included, of whom 630 were treated with IFX and 232 were treated with ADA. Over a median follow-up of 1.8 years after starting biologic therapy, there were no significant differences in the risk of colectomy [adjusted hazard ratio (aHR), 1.87; 95% confidence interval (CI), 0.30-11.63], ER visits (aHR, 1.58; 95% CI, 0.79-3.16), hospitalizations (aHR, 0.83; 95% CI, 0.59-1.17), and corticosteroid use (aHR, 1.16; 95% CI, 0.76-1.78) between IFX and ADA users. These results were stable even when only patients who used biologics for ≥6 months were analyzed. Additionally, these results were unchanged in patients treated with biologic monotherapy or combination therapy with immunomodulators.
CONCLUSION
In this nationwide population-based study, there was no significant difference in the risk of colectomy, ER visits, hospitalizations, and corticosteroid use between IFX and ADA users. Our findings indicate that IFX and ADA have comparable effectiveness in biologic-naïve Korean patients with UC.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018; 16:26–42. PMID: 29422795.
Article2. Ooi CJ, Hilmi I, Banerjee R, Chuah SW, Ng SC, Wei SC, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn's disease in Asia. J Gastroenterol Hepatol. 2019; 34:1296–1315. PMID: 30848854.
Article3. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476. PMID: 16339095.
Article4. Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012; 142:257–265. PMID: 22062358.
Article5. Thorlund K, Druyts E, Mills EJ, Fedorak RN, Marshall JK. Adalimumab versus infliximab for the treatment of moderate to severe ulcerative colitis in adult patients naïve to anti-TNF therapy: an indirect treatment comparison meta-analysis. J Crohns Colitis. 2014; 8:571–581. PMID: 24491514.
Article6. Stidham RW, Lee TC, Higgins PD, Deshpande AR, Sussman DA, Singal AG, et al. Systematic review with network meta-analysis: the efficacy of anti-tumour necrosis factor-alpha agents for the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2014; 39:660–671. PMID: 24506179.
Article7. Mao EJ, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2017; 45:3–13. PMID: 27862107.
Article8. Ananthakrishnan AN, Cagan A, Cai T, Gainer VS, Shaw SY, Savova G, et al. Comparative effectiveness of infliximab and adalimumab in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2016; 22:880–885. PMID: 26933751.
Article9. Sandborn WJ, Sakuraba A, Wang A, Macaulay D, Reichmann W, Wang S, et al. Comparison of real-world outcomes of adalimumab and infliximab for patients with ulcerative colitis in the United States. Curr Med Res Opin. 2016; 32:1233–1241. PMID: 26986449.
Article10. Singh S, Heien HC, Sangaralingham LR, Schilz SR, Kappelman MD, Shah ND, et al. Comparative effectiveness and safety of infliximab and adalimumab in patients with ulcerative colitis. Aliment Pharmacol Ther. 2016; 43:994–1003. PMID: 26991059.
Article11. Singh S, Andersen NN, Andersson M, Loftus EV Jr, Jess T. Comparison of infliximab and adalimumab in biologic-naive patients with ulcerative colitis: a nationwide Danish cohort study. Clin Gastroenterol Hepatol. 2017; 15:1218–1225. PMID: 27913244.12. Di Domenicantonio R, Trotta F, Cascini S, Agabiti N, Kohn A, Gasbarrini A, et al. Population-based cohort study on comparative effectiveness and safety of biologics in inflammatory bowel disease. Clin Epidemiol. 2018; 10:203–213. PMID: 29440933.
Article13. Gies N, Kroeker KI, Wong K, Fedorak RN. Treatment of ulcerative colitis with adalimumab or infliximab: long-term follow-up of a single-centre cohort. Aliment Pharmacol Ther. 2010; 32:522–528. PMID: 20500733.
Article14. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119. PMID: 27175111.
Article15. Yen HH, Weng MT, Tung CC, Wang YT, Chang YT, Chang CH, et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide populationbased study. Intest Res. 2019; 17:54–62. PMID: 30449079.
Article16. Jung YS, Han M, Kim WH, Park S, Cheon JH. Incidence and clinical outcomes of inflammatory bowel disease in South Korea, 2011-2014: a nationwide population-based study. Dig Dis Sci. 2017; 62:2102–2112. PMID: 28593437.
Article17. Jung YS, Han M, Park S, Kim WH, Cheon JH. Cancer risk in the early stages of inflammatory bowel disease in Korean patients: a nationwide population-based study. J Crohns Colitis. 2017; 11:954–962. PMID: 28333358.
Article18. Han M, Jung YS, Cheon JH, Park S. Regional variations in the use of biologics and immunomodulators among Korean patients with inflammatory bowel diseases. J Gastroenterol Hepatol. 2019; 34:1166–1174. PMID: 30672608.
Article19. Mizoshita T, Katano T, Tanida S, Hirano A, Miyaki T, Ozeki K, et al. Prospective comparison of preference and efficacy of adalimumab and infliximab for treating ulcerative colitis naive to antitumor necrosis factor therapy. Medicine (Baltimore). 2017; 96:e7800. PMID: 28796080.
Article20. Danese S, Fiorino G, Peyrin-Biroulet L, Lucenteforte E, Virgili G, Moja L, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med. 2014; 160:704–711. PMID: 24842416.21. Cholapranee A, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn's disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017; 45:1291–1302. PMID: 28326566.
Article22. Nam GE, Park HS. Perspective on diagnostic criteria for obesity and abdominal obesity in Korean adults. J Obes Metab Syndr. 2018; 27:134–142. PMID: 31089555.
Article23. Chandradas S, Khalili H, Ananthakrishnan A, Wayman C, Reidel W, Waalen J, et al. Does obesity influence the risk of Clostridium difficile infection among patients with ulcerative colitis? Dig Dis Sci. 2018; 63:2445–2450. PMID: 29779082.
Article24. Seminerio JL, Koutroubakis IE, Ramos-Rivers C, Hashash JG, Dudekula A, Regueiro M, et al. Impact of obesity on the management and clinical course of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015; 21:2857–2863. PMID: 26241001.
Article25. Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in inflammatory bowel disease: a marker of less severe disease. Dig Dis Sci. 2015; 60:2436–2445. PMID: 25799938.
Article26. Bultman E, de Haar C, van Liere-Baron A, Verhoog H, West RL, Kuipers EJ, et al. Predictors of dose escalation of adalimumab in a prospective cohort of Crohn's disease patients. Aliment Pharmacol Ther. 2012; 35:335–341. PMID: 22191671.
Article27. Bhalme M, Sharma A, Keld R, Willert R, Campbell S. Does weight-adjusted anti-tumour necrosis factor treatment favour obese patients with Crohn's disease? Eur J Gastroenterol Hepatol. 2013; 25:543–549. PMID: 23337170.
Article28. Bond A, Asher R, Jackson R, Sager K, Martin K, Kneebone A, et al. Comparative analysis of the influence of clinical factors including BMI on adalimumab and infliximab trough levels. Eur J Gastroenterol Hepatol. 2016; 28:271–276. PMID: 26657455.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Long-term Outcomes of Infliximab versus Adalimumab Treatment in Biologic-Naïve Patients with Ulcerative Colitis
- Golimumab Therapy in Ulcerative Colitis
- Real-World Incidence of Suboptimal Response to Anti-Tumor Necrosis Factor Therapy for Ulcerative Colitis: A Nationwide Population-Based Study
- What to do when traditional rescue therapies fail in acute severe ulcerative colitis
- Infliximab versus Adalimumab, Which One Is Better for Ulcerative Colitis?