Int J Stem Cells.
2019 Nov;12(3):388-399. 10.15283/ijsc19047.
Improvement of Human Sperm Vacuolization and DNA Fragmentation Co-Cultured with Adipose-Derived Mesenchymal Stem Cell Secretome: In Vitro Effect
- Affiliations
-
- 1Azoury IVF Clinic, Mount Lebanon Hospital, Beirut, Lebanon.
- 2Regenerative Medicine and Inflammation Laboratory, Faculty of Medicine, Saint-Joseph University, Beirut, Lebanon.
- 3Faculty of Public Health II, Lebanese University, Beirut, Lebanon.
- 4Department of Obstetrics and Gynecology, American University of Beirut Medical Center, Beirut, Lebanon.
- 5OB-GYN Department, Inova Fairfax Hospital, Falls Church, VI, USA.
- 6Neuroscience Research Science, Faculty of Medicine, Lebanese University, Beirut, Lebanon. nada.aladdin@gmail.com
- KMID: 2465879
- DOI: http://doi.org/10.15283/ijsc19047
Abstract
- BACKGROUND AND OBJECTIVES
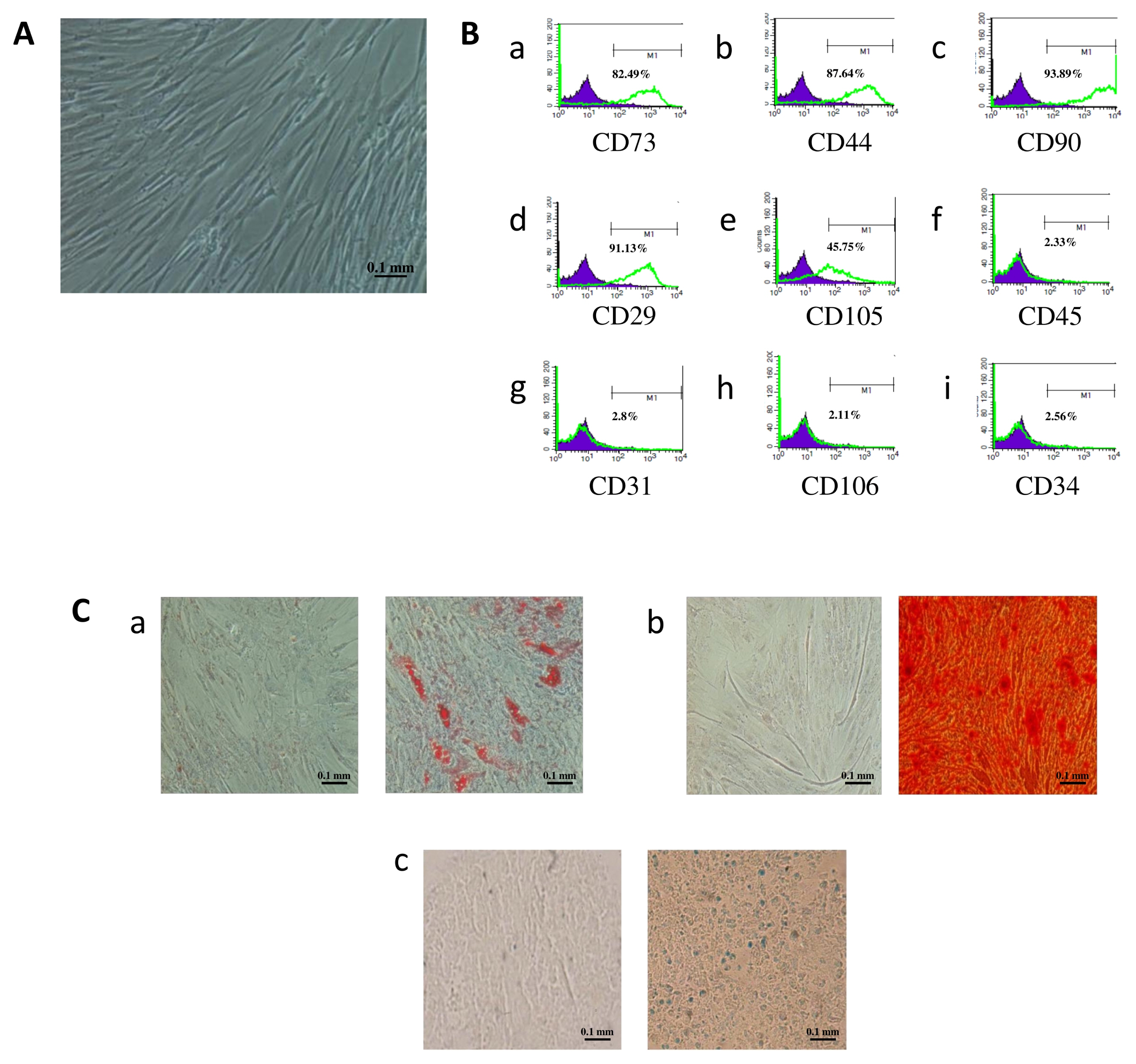
Oxidative stress (OS) is known to be an important factor of male infertility. Adipose-derived mesenchymal stem cells (AD-MSCs) are known to have immune-modulatory and anti-oxidant effects through their secretions, hence raising the idea of their potential benefit to improve sperm parameters. This study aims at investigating the effect of AD-MSCs conditioned medium (CM) on human sperm parameters in the presence and absence of H2O2-induced OS.
METHODS AND RESULTS
Sperm samples were collected from 30 healthy men and divided into two groups: non-stressed and H2O2-stressed. Isolated AD-MSCs from healthy donors undergoing liposuction were cultured and CM was collected at 24, 48 and 72 h. Both sperm groups were cultured with CM and a time course was performed followed by an evaluation of sperm parameters. The incubation of non-stressed and stressed sperm samples with AD-MSCs-CM for 24 h was found to have the optimum impact on sperm vacuolization, DNA fragmentation and OS levels in comparison to other incubation timings, while preserving motility, viability and morphology of cells. Incubation with CM improved all sperm parameters except morphology in comparison to the non-treated group, with the best effect noted with CM collected at 24 h rather than 48 or 72 h for sperm vacuolization and DNA fragmentation. When compared to fresh semen parameters (T0), samples cultured with CM 24 h showed a significant decrease in sperm vacuolization and DNA fragmentation while keeping other parameters stable.
CONCLUSIONS
AD-MSCSs-CM improves sperm quality, and hence can be used in treating infertility and subsequently enhancing IVF outcomes.
MeSH Terms
Figure
Reference
-
References
1. Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015; 21:411–426. DOI: 10.1093/humupd/dmv016. PMID: 25801630.
Article2. Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015; 13:37. DOI: 10.1186/s12958-015-0032-1. PMID: 25928197. PMCID: PMC4424520.
Article3. Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014; 32:1–17. DOI: 10.5534/wjmh.2014.32.1.1. PMID: 24872947. PMCID: PMC4026229.
Article4. Herrero C, Pérez-Simón JA. Immunomodulatory effect of mesenchymal stem cells. Braz J Med Biol Res. 2010; 43:425–430. DOI: 10.1590/S0100-879X2010007500033. PMID: 20490429.
Article5. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317. DOI: 10.1080/14653240600855905. PMID: 16923606.
Article6. Xu J, Zgheib C, Shear D, Li G, Hu J, Liechty KW. Mesenchymal stem cell treatment modulates oxidative stress and improves diabetic wound healing through increased expression of glutathione peroxidase 4. J Am Coll Surg. 2014; 219:e15. DOI: 10.1016/j.jamcollsurg.2014.07.426.
Article7. Cejka C, Holan V, Trosan P, Zajicova A, Javorkova E, Cejkova J. The favorable effect of mesenchymal stem cell treatment on the antioxidant protective mechanism in the corneal epithelium and renewal of corneal optical properties changed after alkali burns. Oxid Med Cell Longev. 2016; 2016:5843809. DOI: 10.1155/2016/5843809. PMID: 27057279. PMCID: PMC4736412.
Article8. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006; 119:2204–2213. DOI: 10.1242/jcs.02932. PMID: 16684817.
Article9. Hoogduijn MJ, Dor FJ. Mesenchymal stem cells: are we ready for clinical application in transplantation and tissue regeneration? Front Immunol. 2013; 4:144. DOI: 10.3389/fimmu.2013.00144. PMID: 23781219. PMCID: PMC3678105.
Article10. Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014; 2014:965849. DOI: 10.1155/2014/965849. PMID: 25530971. PMCID: PMC4229962.
Article11. Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, Ma ZJ. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015; 6:55. DOI: 10.1186/s13287-015-0066-5. PMID: 25884704. PMCID: PMC4453294.
Article12. Fazaeli H, Faeze D, Naser K, Maryam S, Mohammad M, Mahdieh G, Reza TQ. Introducing of a new experimental method in semen preparation: supernatant product of adipose tissue: derived mesenchymal stem cells (SPAS). JFIV Reprod Med Genet. 2016; 4:178. DOI: 10.4172/2375-4508.1000178.
Article13. Fazaeli H, Davoodi F, Kalhor N, Tabatabaii Qomi R. The effect of supernatant product of adipose tissue derived mesenchymal stem cells and density gradient centrifugation preparation methods on pregnancy in intrauterine insemination cycles: an RCT. Int J Reprod Biomed (Yazd). 2018; 16:199–208. DOI: 10.29252/ijrm.16.3.199. PMID: 29766151. PMCID: PMC5944442.
Article14. World Health Organization, Department of Reproductive Health and Research. WHO laboratory manual for the examination and processing of human semen [Internet]. Geneva: WHO;2010. [cited 2018 Sep 26]. Available from: http://www.who.int/reproductivehealth/publications/infertility/9789241547789/en/.15. Esfandiari N, Sharma RK, Saleh RA, Thomas AJ Jr, Agarwal A. Utility of the nitroblue tetrazolium reduction test for assessment of reactive oxygen species production by seminal leukocytes and spermatozoa. J Androl. 2003; 24:862–870. DOI: 10.1002/j.1939-4640.2003.tb03137.x. PMID: 14581512.
Article16. Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters: an evidence based review. Int J Reprod Biomed (Yazd). 2016; 14:729–736. DOI: 10.29252/ijrm.14.12.729. PMID: 28066832. PMCID: PMC5203687.
Article17. Kovac JR, Lipshultz LI. Use of testicular sperm to combat the negative effects of DNA fragmentation. Asian J Androl. 2016; 18:434. DOI: 10.4103/1008-682X.179158. PMID: 27048786. PMCID: PMC4854097.
Article18. Alahmar AT. The effects of oral antioxidants on the semen of men with idiopathic oligoasthenoteratozoospermia. Clin Exp Reprod Med. 2018; 45:57–66. DOI: 10.5653/cerm.2018.45.2.57. PMID: 29984205. PMCID: PMC6030611.
Article19. Kim Y, Jo SH, Kim WH, Kweon OK. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res Ther. 2015; 6:229. DOI: 10.1186/s13287-015-0236-5. PMID: 26612085. PMCID: PMC4660672.
Article20. Lanza C, Morando S, Voci A, Canesi L, Principato MC, Serpero LD, Mancardi G, Uccelli A, Vergani L. Neuroprotective mesenchymal stem cells are endowed with a potent antioxidant effect in vivo. J Neurochem. 2009; 110:1674–1684. DOI: 10.1111/j.1471-4159.2009.06268.x. PMID: 19619133.
Article21. Hassan AI, Alam SS. Evaluation of mesenchymal stem cells in treatment of infertility in male rats. Stem Cell Res Ther. 2014; 5:131. DOI: 10.1186/scrt521. PMID: 25422144. PMCID: PMC4528845.
Article22. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017; 6:1445–1451. DOI: 10.1002/sctm.17-0051. PMID: 28452204. PMCID: PMC5689741.
Article23. Zini A, Finelli A, Phang D, Jarvi K. Influence of semen processing technique on human sperm DNA integrity. Urology. 2000; 56:1081–1084. DOI: 10.1016/S0090-4295(00)00770-6. PMID: 11113773.
Article24. Zhou BR, Xu Y, Guo SL, Xu Y, Wang Y, Zhu F, Permatasari F, Wu D, Yin ZQ, Luo D. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. Biomed Res Int. 2013; 2013:519126. DOI: 10.1155/2013/519126. PMID: 24381938. PMCID: PMC3867954.
Article25. Sevivas N, Teixeira FG, Portugal R, Araújo L, Carriço LF, Ferreira N, Vieira da Silva M, Espregueira-Mendes J, Anjo S, Manadas B, Sousa N, Salgado AJ. Mesenchymal stem cell secretome: a potential tool for the prevention of muscle degenerative changes associated with chronic rotator cuff tears. Am J Sports Med. 2017; 45:179–188. DOI: 10.1177/0363546516657827. PMID: 27501832.
Article26. Prihatno SA, Padeta I, Larasati AD, Sundari B, Hidayati A, Fibrianto YH, Budipitojo T. Effects of secretome on cis-platin-induced testicular dysfunction in rats. Vet World. 2018; 11:1349–1356. DOI: 10.14202/vetworld.2018.1349-1356. PMID: 30410245. PMCID: PMC6200560.
Article27. Mokarizadeh A, Rezvanfar MA, Dorostkar K, Abdollahi M. Mesenchymal stem cell derived microvesicles: trophic shuttles for enhancement of sperm quality parameters. Reprod Toxicol. 2013; 42:78–84. DOI: 10.1016/j.reprotox.2013.07.024. PMID: 23958892.
Article28. Catizone A, Ricci G, Galdieri M. Functional role of hepatocyte growth factor receptor during sperm maturation. J Androl. 2002; 23:911–918. PMID: 12399538.29. Iyibozkurt AC, Balcik P, Bulgurcuoglu S, Arslan BK, Attar R, Attar E. Effect of vascular endothelial growth factor on sperm motility and survival. Reprod Biomed Online. 2009; 19:784–788. DOI: 10.1016/j.rbmo.2009.09.019. PMID: 20031017.
Article30. Saucedo L, Buffa GN, Rosso M, Guillardoy T, Góngora A, Munuce MJ, Vazquez-Levin MH, Marín-Briggiler C. Fibroblast growth factor receptors (FGFRs) in human sperm: expression, functionality and involvement in motility regulation. PLoS One. 2015; 10:e0127297. DOI: 10.1371/journal.pone.0127297. PMID: 25970615. PMCID: PMC4430232.
Article31. Selvaraju S, Krishnan BB, Archana SS, Ravindra JP. IGF1 stabilizes sperm membrane proteins to reduce cryoinjury and maintain post-thaw sperm motility in buffalo (Bubalus bubalis) spermatozoa. Cryobiology. 2016; 73:55–62. DOI: 10.1016/j.cryobiol.2016.05.012. PMID: 27256665.
Article32. Saeednia S, Bahadoran H, Amidi F, Asadi MH, Naji M, Fallahi P, Nejad NA. Nerve growth factor in human semen: effect of nerve growth factor on the normozoospermic men during cryopreservation process. Iran J Basic Med Sci. 2015; 18:292–299. PMID: 25945243. PMCID: PMC4414996.33. Saeednia S, Shabani Nashtaei M, Bahadoran H, Aleyasin A, Amidi F. Effect of nerve growth factor on sperm quality in asthenozoosprmic men during cryopreservation. Reprod Biol Endocrinol. 2016; 14:29. DOI: 10.1186/s12958-016-0163-z. PMID: 27233989. PMCID: PMC4884433.
Article34. Kim J, Lee S, Jeon B, Jang W, Moon C, Kim S. Protection of spermatogenesis against gamma ray-induced damage by granulocyte colony-stimulating factor in mice. Andrologia. 2011; 43:87–93. DOI: 10.1111/j.1439-0272.2009.01023.x. PMID: 21382061.
Article35. Mullaney BP, Skinner MK. Transforming growth factor-beta (beta 1, beta 2, and beta 3) gene expression and action during pubertal development of the seminiferous tubule: potential role at the onset of spermatogenesis. Mol Endocrinol. 1993; 7:67–76. PMID: 8446109.
Article36. Białas M, Fiszer D, Rozwadowska N, Kosicki W, Jedrzejczak P, Kurpisz M. The role of IL-6, IL-10, TNF-alpha and its receptors TNFR1 and TNFR2 in the local regulatory system of normal and impaired human spermatogenesis. Am J Reprod Immunol. 2009; 62:51–59. DOI: 10.1111/j.1600-0897.2009.00711.x. PMID: 19527232.
Article37. Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod. 2007; 22:2928–2935. DOI: 10.1093/humrep/dem281. PMID: 17855405.
Article38. Kisa Ü, Murad M, Ferhat M, Ça O. Seminal plasma transforming growth factor-β (TGF-β) and epidermal growth factor (EGF) levels in patients with varicocele. Turk J Med Sci. 2008; 38:105–110.39. Kemp K, Hares K, Mallam E, Heesom KJ, Scolding N, Wilkins A. Mesenchymal stem cell-secreted superoxide dismutase promotes cerebellar neuronal survival. J Neurochem. 2010; 114:1569–1580. DOI: 10.1111/j.1471-4159.2009.06553.x. PMID: 20028455.
Article40. Zhao J, Dong X, Hu X, Long Z, Wang L, Liu Q, Sun B, Wang Q, Wu Q, Li L. Zinc levels in seminal plasma and their correlation with male infertility: a systematic review and meta-analysis. Sci Rep. 2016; 6:22386. DOI: 10.1038/srep22386. PMID: 26932683. PMCID: PMC4773819.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Adipose-derived stem cells: characterization and clinical application
- Liver Regenerating Potential of the Secretome Obtained from Adipose-derived Stem Cells Cultured under the Hypoxic Environment
- Adipose Tissue Derived Mesenchymal Stem Cells
- Isolation of Secretome with Enhanced Antifibrotic Properties from miR-214-Transfected Adipose-Derived Stem Cells