Korean J Physiol Pharmacol.
2019 Nov;23(6):501-508. 10.4196/kjpp.2019.23.6.501.
Effects of rosmarinic acid on immunoregulatory activity and hepatocellular carcinoma cell apoptosis in H22 tumor-bearing mice
- Affiliations
-
- 1Department of Pharmacy, Guangxi International Zhuang Medicine Hospital, Guangxi 530200, China. mosquito1106@foxmail.com
- 2Department of Pharmacy, Nanning First People's Hospital, Guangxi 530022, China.
- 3Department of Pharmacology, Guangxi Medical University, Guangxi 530021, China. jiangweizhe6812@163.com
- KMID: 2461043
- DOI: http://doi.org/10.4196/kjpp.2019.23.6.501
Abstract
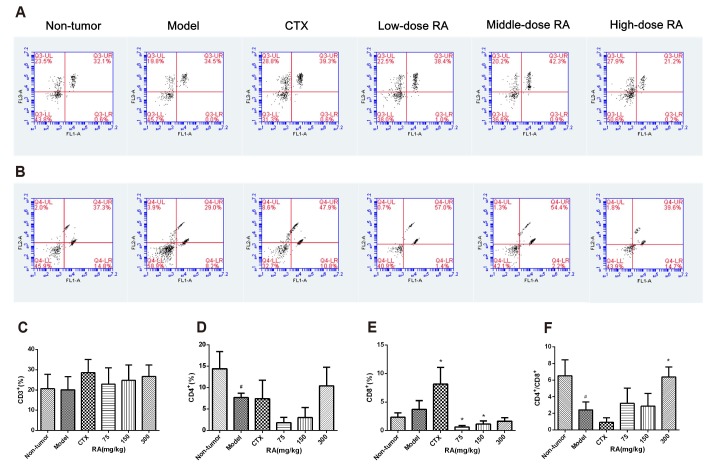
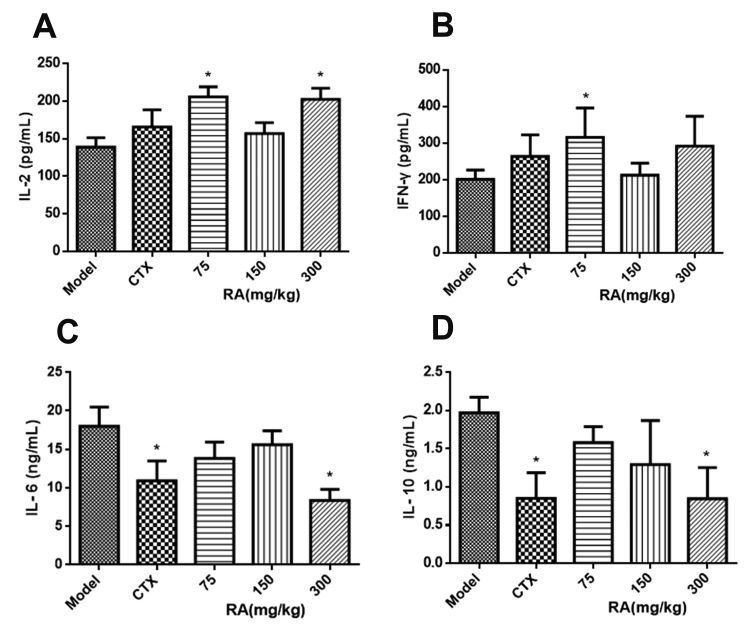
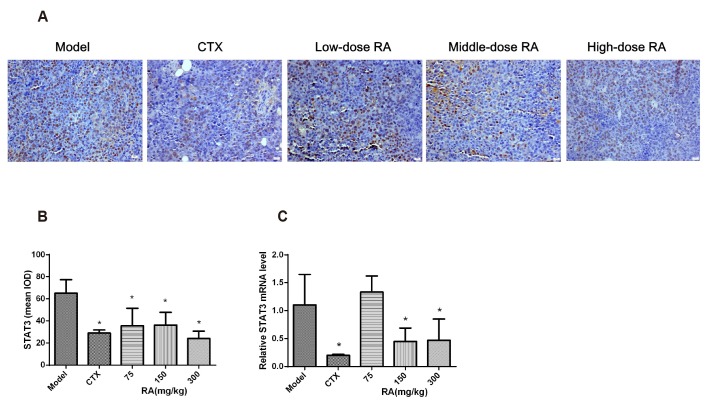
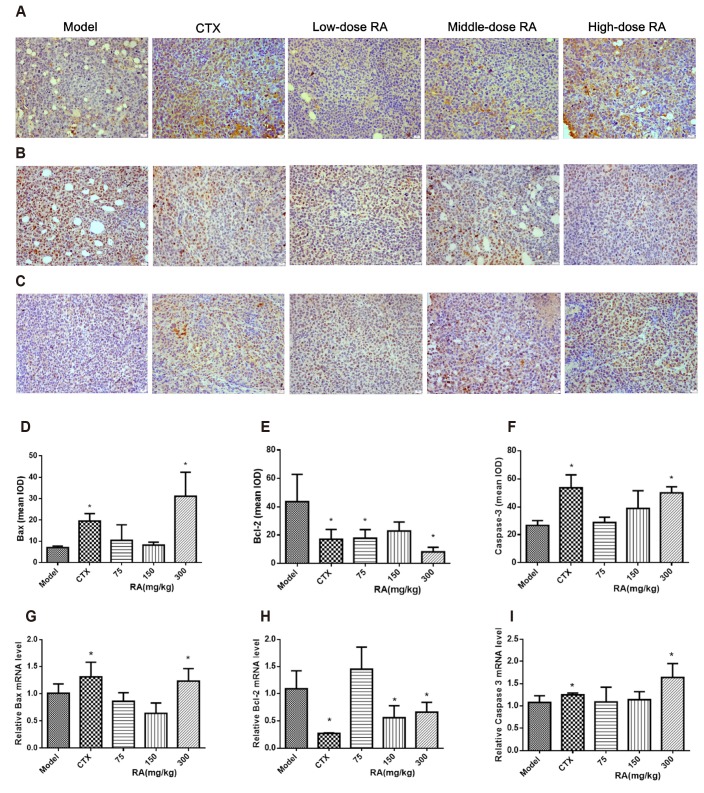
- Rosmarinic acid (RA) is a natural polyphenolic compound that exists in many medicinal species of Boraginaceae and Lamiaceae. The previous studies have revealed that RA had therapeutic effects on hepatocellular carcinoma (HCC) in the H22-xenograft models by inhibiting the inflammatory cytokines and NF-κB p65 pathway in the tumor microenvironment. However, its molecular mechanisms of immunoregulation and pro-apoptotic effect in HCC have not been fully explored. In the present study, RA at 75, 150, and 300 mg/kg was given to H22 tumor-bearing mice via gavage once a day for 10 days. The results showed that RA can effectively inhibit the tumor growth through regulating the ratio of CD4âº/CD8⺠and the secretion of interleukin (IL)-2 and interferon-γ, inhibiting the expressions of IL-6, IL-10 and signal transducer and activator of transcription 3, thereby up-regulating Bax and Caspase-3 and down-regulating Bcl-2. The underlying mechanisms involved regulation of immune response and induction of HCC cell apoptosis. These results may provide a more comprehensive perspective to clarify the anti-tumor mechanism of RA in HCC.
MeSH Terms
Figure
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. PMID: 25651787.
Article2. Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010; 30:3–16. PMID: 20175029.
Article3. Nabavi SF, Tenore GC, Daglia M, Tundis R, Loizzo MR, Nabavi SM. The cellular protective effects of rosmarinic acid: from bench to bedside. Curr Neurovasc Res. 2015; 12:98–105. PMID: 25578431.
Article4. Rocha J, Eduardo-Figueira M, Barateiro A, Fernandes A, Brites D, Bronze R, Duarte CM, Serra AT, Pinto R, Freitas M, Fernandes E, Silva-Lima B, Mota-Filipe H, Sepodes B. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin Pharmacol Toxicol. 2015; 116:398–413. PMID: 25287116.5. Karthikkumar V, Sivagami G, Viswanathan P, Nalini N. Rosmarinic acid inhibits DMH-induced cell proliferation in experimental rats. J Basic Clin Physiol Pharmacol. 2015; 26:185–200. PMID: 25210763.
Article6. Cao W, Hu C, Wu L, Xu L, Jiang W. Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-κB signaling in H22 tumor-bearing mice. J Pharmacol Sci. 2016; 132:131–137. PMID: 27707649.
Article7. Chan T, Wiltrout RH, Weiss JM. Immunotherapeutic modulation of the suppressive liver and tumor microenvironments. Int Immunopharmacol. 2011; 11:879–889. PMID: 21241810.
Article8. Wirth TC. Spontaneous and therapeutic immune responses in hepatocellular carcinoma: implications for current and future immunotherapies. Expert Rev Gastroenterol Hepatol. 2014; 8:101–110. PMID: 24410473.
Article9. Xiang M, Su H, Hong Z, Yang T, Shu G. Chemical composition of total flavonoids from Polygonum amplexicaule and their proapoptotic effect on hepatocellular carcinoma cells: Potential roles of suppressing STAT3 signaling. Food Chem Toxicol. 2015; 80:62–71. PMID: 25754378.
Article10. Subramaniam A, Shanmugam MK, Perumal E, Li F, Nachiyappan A, Dai X, Swamy SN, Ahn KS, Kumar AP, Tan BK, Hui KM, Sethi G. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys Acta. 2013; 1835:46–60. PMID: 23103770.
Article11. Venkatachalam K, Gunasekaran S, Jesudoss VA, Namasivayam N. The effect of rosmarinic acid on 1,2-dimethylhydrazine induced colon carcinogenesis. Exp Toxicol Pathol. 2013; 65:409–418. PMID: 22236574.
Article12. Han S, Yang S, Cai Z, Pan D, Li Z, Huang Z, Zhang P, Zhu H, Lei L, Wang W. Anti-Warburg effect of rosmarinic acid via miR-155 in gastric cancer cells. Drug Des Devel Ther. 2015; 9:2695–2703.13. Xu Y, Jiang Z, Ji G, Liu J. Inhibition of bone metastasis from breast carcinoma by rosmarinic acid. Planta Med. 2010; 76:956–962. PMID: 20157877.
Article14. Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007; 19:203–208. PMID: 17292599.
Article15. Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011; 7:651–658. PMID: 21647333.
Article16. Ramzan M, Sturm N, Decaens T, Bioulac-Sage P, Bancel B, Merle P, Tran Van, Slama R, Letoublon C, Zarski JP, Jouvin-Marche E, Marche PN, Leroy V. Liver-infiltrating CD8(+) lymphocytes as prognostic factor for tumour recurrence in hepatitis C virus-related hepatocellular carcinoma. Liver Int. 2016; 36:434–444. PMID: 26215124.
Article17. Wu D, Wei S, Liu B, Wu X, Feng Y, Luo C, Ju Y, Liang J. Effect of immune suppression on metastasis in a patient with hepatocellular carcinoma metastasized to the colon and stomach: a case report. Exp Ther Med. 2016; 11:1741–1747. PMID: 27168796.
Article18. Schmidt N, Neumann-Haefelin C, Thimme R. Cellular immune responses to hepatocellular carcinoma: lessons for immunotherapy. Dig Dis. 2012; 30:483–491. PMID: 23108304.
Article19. Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White JM, Sheehan KC, Schreiber RD. Interferon-gamma and cancer immunoediting. Immunol Res. 2005; 32:231–245. PMID: 16106075.20. Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004; 28:109–123. PMID: 15473953.
Article21. He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011; 21:159–168. PMID: 21187858.
Article22. Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: a review. Int J Cancer. 2016; 138:2570–2578. PMID: 26559373.
Article23. Zhao H, Guo Y, Li S, Han R, Ying J, Zhu H, Wang Y, Yin L, Han Y, Sun L, Wang Z, Lin Q, Bi X, Jiao Y, Jia H, Zhao J, Huang Z, Li Z, Zhou J, Song W, et al. A novel anti-cancer agent Icaritin suppresses hepatocellular carcinoma initiation and malignant growth through the IL-6/Jak2/Stat3 pathway. Oncotarget. 2015; 6:31927–31943. PMID: 26376676.
Article24. Ohishi W, Cologne JB, Fujiwara S, Suzuki G, Hayashi T, Niwa Y, Akahoshi M, Ueda K, Tsuge M, Chayama K. Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int J Cancer. 2014; 134:154–163. PMID: 23784949.
Article25. Chan SL, Mo FK, Wong CS, Chan CM, Leung LK, Hui EP, Ma BB, Chan AT, Mok TS, Yeo W. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer. 2012; 118:3984–3992. PMID: 22180222.
Article26. Karabulut AB, Karadag N, Gurocak S, Kiran T, Tuzcu M, Sahin K. Apricot attenuates oxidative stress and modulates of Bax, Bcl-2, caspases, NFκ-B, AP-1, CREB expression of rats bearing DMBA-induced liver damage and treated with a combination of radiotherapy. Food Chem Toxicol. 2014; 70:128–133. PMID: 24819963.
Article27. Shao J, Meng Q, Li Y. Theaflavins suppress tumor growth and metastasis via the blockage of the STAT3 pathway in hepatocellular carcinoma. Onco Targets Ther. 2016; 9:4265–4275. PMID: 27478384.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Expression of Proliferating Cell Nuclear Antigen and Apoptosis of the Hepatocellular Carcinoma in Human and Hepatitis B Virus X Transgenic Mice
- P53 expression in hepatocellular carcinoma: influence on the radiotherapeutic response of the hepatocellular carcinoma
- Induction of apoptosis by bile acids in HepG2 human hepatocellular carcinoma cells
- Analysis of Cell Proliferative Activity, p53 Protein Overexpression and Apoptosis in Hepatocellular Carcinoma and Surrounding Nontumorous Liver
- An experimental study on mistletoe extract-induced apoptosis in oral squamous cell carcinoma