Nat Prod Sci.
2019 Sep;25(3):208-214. 10.20307/nps.2019.25.3.208.
The Effect of Trigonella foenum-graceum L. (Fenugreek) Towards Collagen Type I Alpha 1 (COL1A1) and Collagen Type III Alpha 1 (COL3A1) on Postmenopausal Woman's Fibroblast
- Affiliations
-
- 1Department of Dermatology and Venereology, Faculty of Medicine, Universitas Indonesia - Dr. Cipto Mangunkusumo General Hospital, Indonesia. nadiayusharyahya@yahoo.com
- 2Department of Physiology, Faculty of Medicine University of YARSI, Indonesia.
- KMID: 2459961
- DOI: http://doi.org/10.20307/nps.2019.25.3.208
Abstract
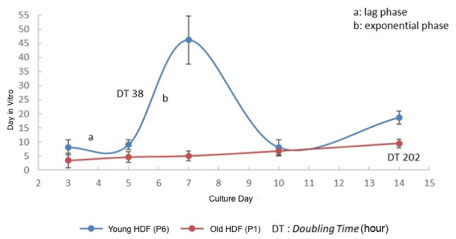
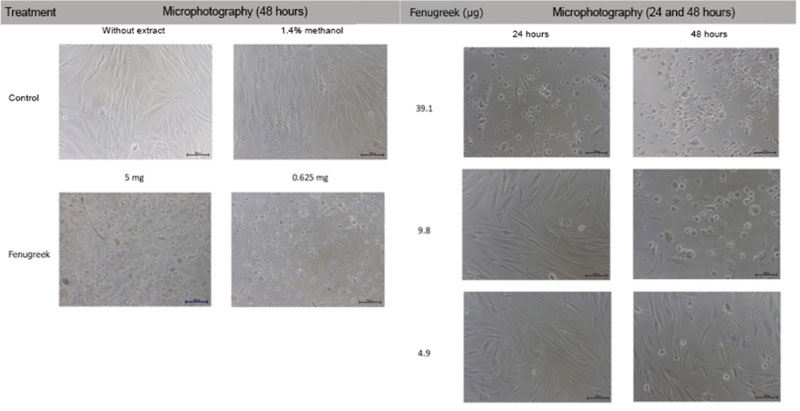
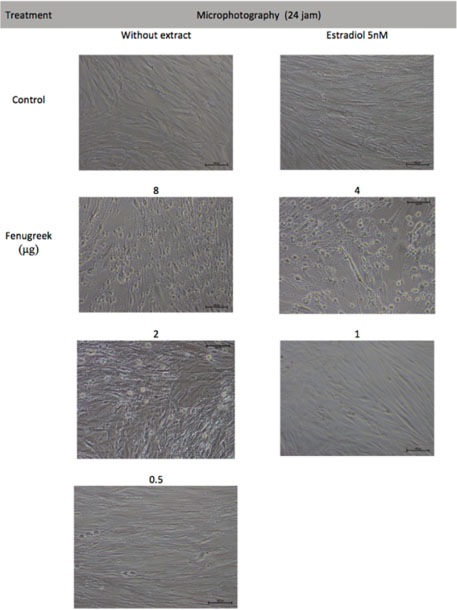
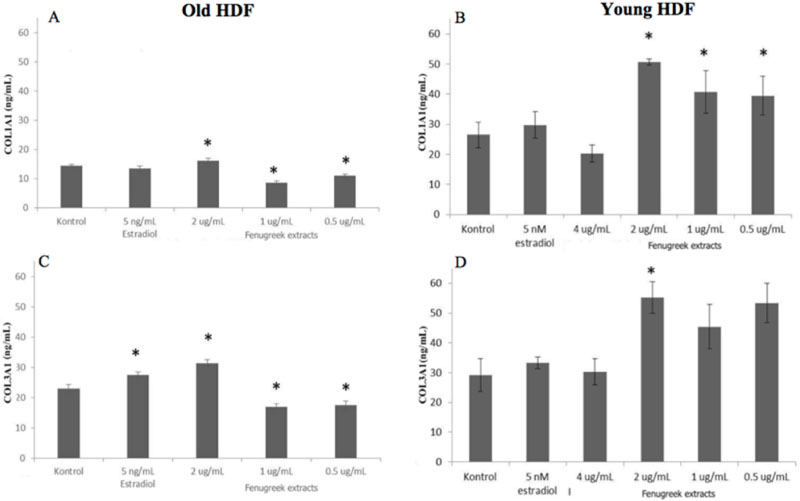
- Trigonella foenum-graceum L. (fenugreek) is a phytoestrogen, a nonsteroidal organic chemical compound from plants which has similar mechanism of action to sex hormone estradiol-17β. This study aims to assess the effectivity of fenugreek seeds extract on collagen type I alpha 1 (COL1A1) and collagen type III alpha 1 (COL3A1) which are both decreased in aging skin and become worsen after menopause. This in vitro experimental study used old human dermal fibroblast from leftover tissue of blepharoplasty on a postmenopausal woman (old HDF). As a control of the fenugreek's ability to trigger collagen production, we used fibroblast from preputium (young HDF). Subsequent to fibroblast isolation and culture, toxicity test was conducted on both old and young HDF by measuring cell viability on fenugreek extract with the concentration of 5 mg/mL to 1.2 µg/mL which will be tested on both HDF to examine COL1A1 and COL3A1 using ELISA, compared to no treatment and 5 nM estradiol. Old HDF showed a 4 times slower proliferation compared to young HDF (p<0.05). Toxicity test revealed fenugreek concentration of 0.5 - 2 µg/mL was non-toxic to both old and young HDF. The most significant fenugreek concentration to increase COL1A1 and COL3A1 secretion was 2 µg/mL (p<0.05).
Keyword
MeSH Terms
Figure
Reference
-
1. Borg M, Brincat S, Camilleri G, Schembri-Wismayer P, Brincat M, Calleja-Agius J. Climateric. 2013; 16:514–521.2. Draelos ZD. J Drugs Dermatol. 2018; 17:1186–1189.3. Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Fertil Steril. 2001; 75:874–878.4. Brincat M, Versi E, Moniz CF, Magos A, de Trafford J, Studd JW. Obstet Gynecol. 1987; 70:123–127.5. Schmidt JB, Binder M, Demschik G, Bieglmayer C, Reiner A. Int J Dermatol. 1996; 35:669–674.6. Thornton MJ. Exp Dermatol. 2002; 11:487–502.
Article7. Affinito P, Palomba S, Sorrentino C, Di Carlo C, Bifulco G, Arienzo MP, Nappi C. . Maturitas. 1999; 33:239–247.8. Varila E, Rantala I, Oikarinen A, Risteli J, Reunala T, Oksanen H, Punnonen R. Br J Obstet Gynaecol. 1995; 102:985–989.9. Rittié L, Kang S, Voorhees JJ, Fisher GJ. Arch Dermatol. 2008; 144:1129–1140.10. Kapuścińska A, Nowak I. Chemik. 2015; 69:154–159.11. Malaivijitnond S. Front Med. 2012; 6:8–21.12. Agustini K, Wiryowidagdo S, Kusmana D. Majalah Ilmu Kefarmasian. 2007; 4:26–36.13. Stevenson S, Thornton J. Clin Interv Aging. 2007; 2:283–297.14. Thornton MJ. Dermatoendocrinol. 2013; 5:264–270.15. Sator PG, Schmidt JB, Rabe T, Zouboulis CC. Exp Dermatol. 2004; 13:36–40.16. Mehrafarin A, Rezazadeh SH, Naghdi BH, Noormohammadi GH, Zand E, Qaderi A. J Med Plants. 2011; 10:6–24.17. Wani SA, Kumar P. Journal of the Saudi Society of Agricultural Science. 2018; 17:97–106.18. Shailajan S, Menon S, Singh A, Mhatre M, Sayed N. Pharm Methods. 2011; 2:157–160.19. Moradi kor N, Didarshetaban MB, Pour HRS. Int J Adv Biol Biom Res. 2013; 1:922–931.20. Akhtar N, Waqas MK, Ahmed M, Saeed T, Murtaza G, Rasool A, Aamir MN, Khan SA, Bhatti NS, Ali A. Trop J Pharm Res. 2010; 9:329–337.21. Gade J, More S, Bhalerao N. Int J Sci Res Pul. 2015; 5:1–4.22. Falla TJ, Zhang L. J Drugs Dermatol. 2010; 9:49–54.23. Subhashini N, Thangathirupathi A, Lavanya N. Int J Pharm Pharm Sci. 2011; 3:96–102.
Article24. Waqas MK, Akhtar N, Ahmad M, Murtaza G, Khan HM, Iqbal M, Rasul A, Bhatti NS. Acta Pol Pharm. 2010; 67:173–178.25. Sreeja S, Anju VS, Sreeja S. Indian J Med Res. 2010; 131:814–819.
Article26. Hadi RS, Kusuma I, Sandra Y. Univ Med. 2014; 33:91–99.27. Kusuma I, Hadi RS. Univ Med. 2013; 32:3–10.28. Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, Minor L. Assay Guidance Manual. U.S.A: Eli Lilly & Company and the National Center for Advancing Translational Sciences;2004. p. 1–25.29. Surazynski A, Jarzabek K, Haczynski J, Laudanski P, Palka J, Wolczynski S. Int J Mol Med. 2003; 12:803–809.30. Wang C, Rong YH, Ning FG, Zhang GA. Afr J Biotechnol. 2011; 10:2524–2529.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Estrogens and Isoflavones on the Collagen Synthesis
- Variable Effect of Estrogen on Fibroblast Proliferation and Collagen Synthesis by Gender and Age
- The Vascular Type Ehlers-Danlos Syndrome Diagnosed after Delivery
- Effect of Glycolic Acid on Collagen Gene Expression in Cultured Human Skin Fibroblasts
- The Effects of Basic Fibroblast Growth Factor(bFGF)on Type I and VII Collagen Gene Expression in Cultured Dermal Fibroblast