Lab Anim Res.
2018 Dec;34(4):317-328. 10.5625/lar.2018.34.4.317.
Comparison of scopolamine-induced cognitive impairment responses in three different ICR stocks
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2College of Veterinary Medicine, Kyungpook National University, Daegu, Korea.
- 3College of Pharmacy, Pusan National University, Busan, Korea.
- 4Exercise Biochemistry Laboratory, Korea National Sport University, Seoul, Korea.
- 5Biomedical Science Institute, Changwon National University, Changwon, Korea. kali71@hanmail.net
- KMID: 2459311
- DOI: http://doi.org/10.5625/lar.2018.34.4.317
Abstract
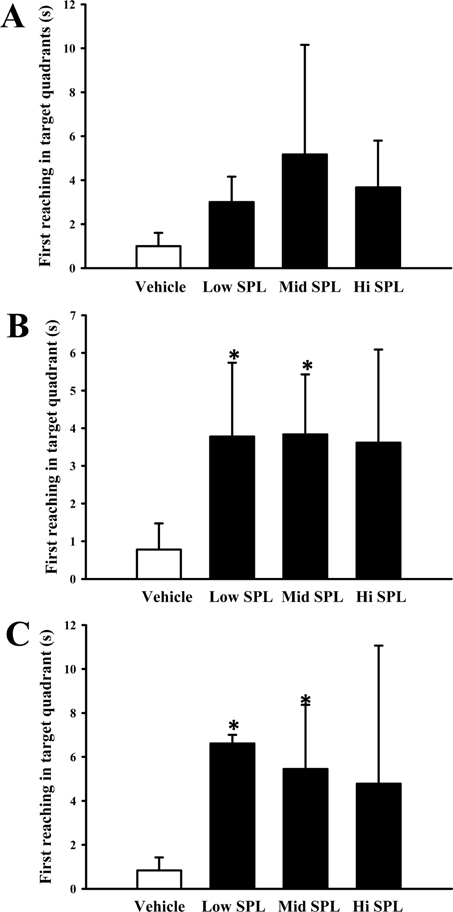
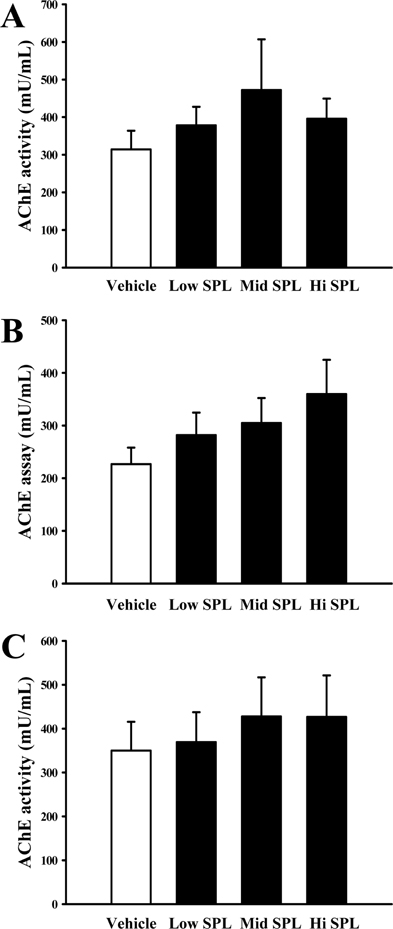
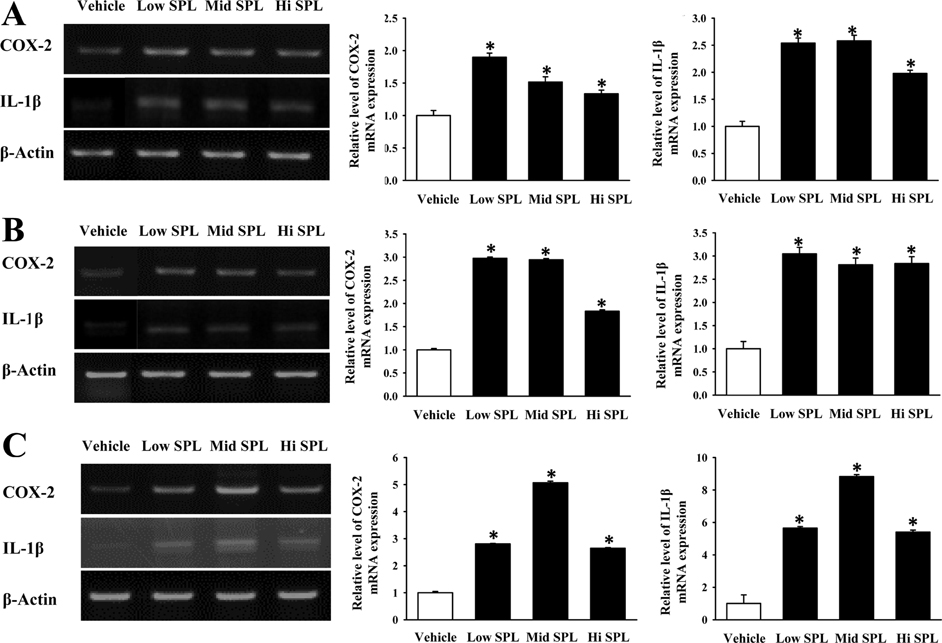
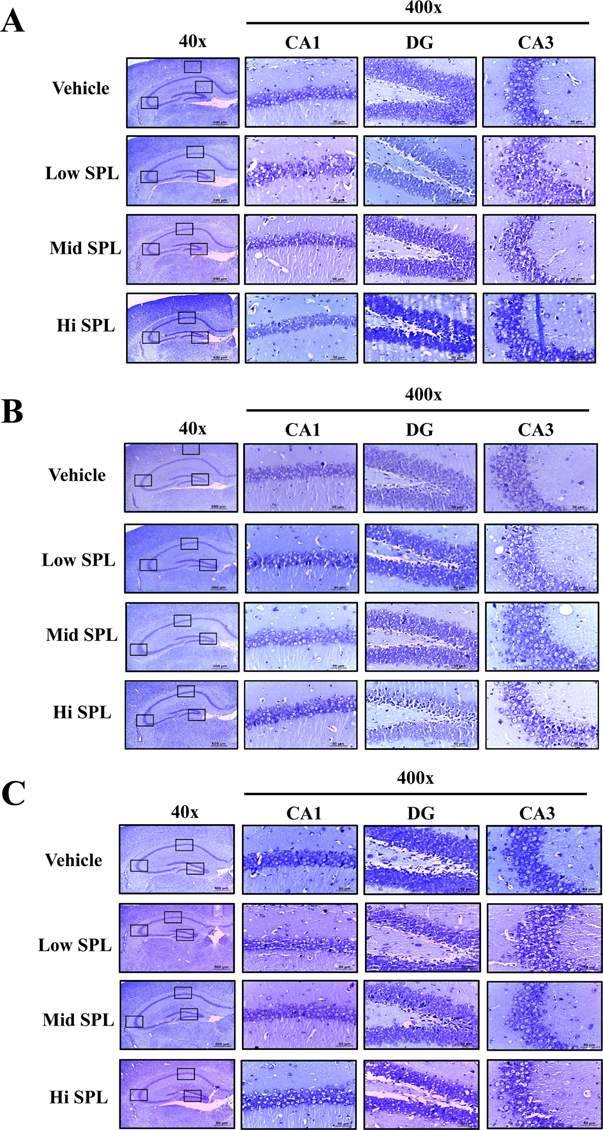
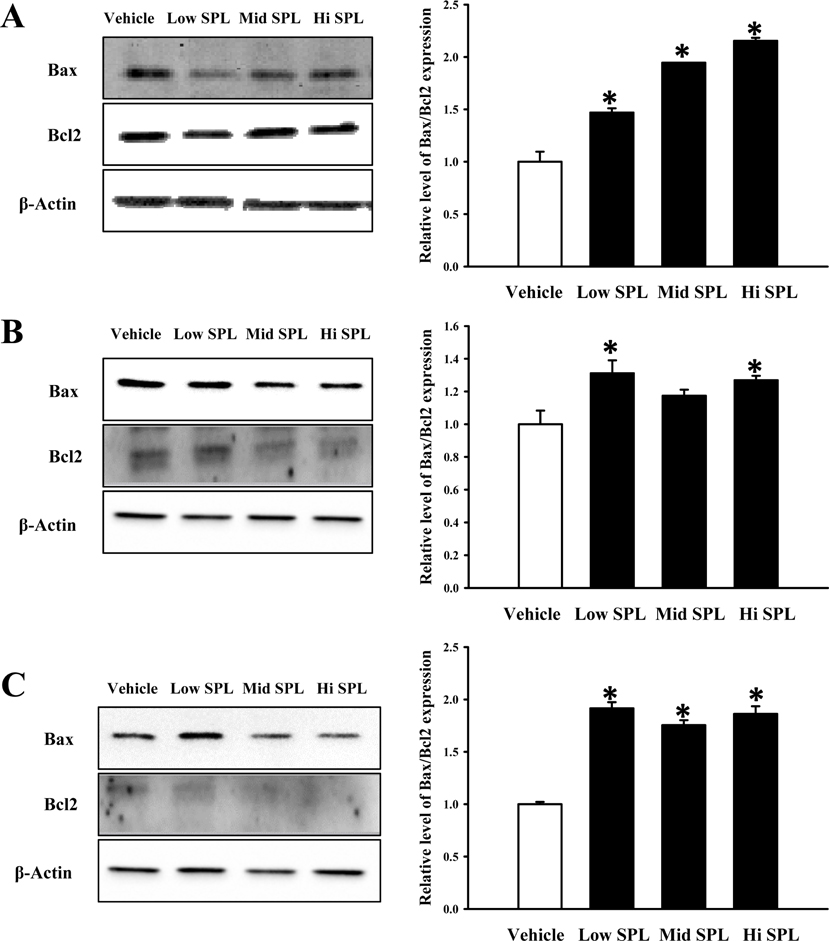
- Cognitive impairment responses are important research topics in the study of degenerative brain diseases as well as in understanding of human mental activities. To compare response to scopolamine (SPL)-induced cognitive impairment, we measured altered parameters for learning and memory ability, inflammatory response, oxidative stress, cholinergic dysfunction and neuronal cell damages, in Korl:ICR stock and two commercial breeder stocks (A:ICR and B:ICR) after relevant SPL exposure. In the water maze test, Korl:ICR showed no significant difference in SPL-induced learning and memory impairment compared to the two different ICRs, although escape latency was increased after SPL exposure. Although behavioral assessment using the manual avoidance test revealed reduced latency in all ICR mice after SPL treatment as compared to Vehicle, no differences were observed between the three ICR stocks. To determine cholinergic dysfunction induction by SPL exposure, activity of acetylcholinesterase (AChE) assessed in the three ICR stocks revealed no difference of acetylcholinesterase activity. Furthermore, low levels of superoxide dismutase (SOD) activity and high levels of inflammatory cytokines in SPL-treated group were maintained in all three ICR stocks, although some variations were observed between the SPLtreated groups. Neuronal cell damages induced by SPL showed similar response in all three ICR stocks, as assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, Nissl staining analysis and expression analyses of apoptosis-related proteins. Thus, the results of this study provide strong evidence that Korl:ICR is similar to the other two ICR. Stocks in response to learning and memory capacity.
Keyword
MeSH Terms
-
Acetylcholinesterase
Animals
Brain Diseases
Cognition Disorders*
Cytokines
DNA Nucleotidylexotransferase
Humans
Learning
Memory
Mice
Mice, Inbred ICR
Neurons
Oxidative Stress
Scopolamine Hydrobromide
Superoxide Dismutase
United Nations
Acetylcholinesterase
Cytokines
DNA Nucleotidylexotransferase
Scopolamine Hydrobromide
Superoxide Dismutase
Figure
Reference
-
1. Brem AK, Ran K, Pascual-Leone A. Learning and memory. Handb Clin Neurol. 2013; 116:693–737.
Article2. Okano H, Hirano T, Balaban E. Learning and memory. Proc Natl Acad Sci U S A. 2000; 97(23):12403–12404.
Article3. Jewart RD, Green J, Lu CJ, Cellar J, Tune LE. Cognitive, behavioral, and physiological changes in Alzheimer disease patients as a function of incontinence medications. Am J Geriatr Psychiatry. 2005; 13(4):324–328.
Article4. Anderson LA, McConnell SR. Cognitive health: an emerging public health issue. Alzheimers Dement. 2007; 3:2 Suppl. S70–S73.
Article5. Akaike A, Takada-Takatori Y, Kume T, Izumi Y. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci. 2010; 40(1-2):211–216.6. Xu Z, Li H, Jin P. Epigenetics-based therapeutics for neurodegenerative disorders. Curr Transl Geriatr Exp Gerontol Rep. 2012; 1(4):229–236.
Article7. Kim DH, Ryu JH. Differential effects of scopolamine on memory processes in the object recognition test and the morris water maze test in Mice. Biomol Ther. 2008; 16(3):173–178.
Article8. Vannucchi MG, Scali C, Kopf SR, Pepeu G, Casamenti F. Selective muscarinic antagonists differentially affect in vivo acetylcholine release and memory performances of young and aged rats. Neuroscience. 1997; 79(3):837–846.
Article9. Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, Jo TH, Park YI, Lee CK, Kim YB, Lee SY, Jang CG. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol. 2010; 649(1-3):210–217.
Article10. Lee YK, Yuk DY, Kim TI, Kim YH, Kim KT, Kim KH, Lee BJ, Nam SY, Hong JT. Protective effect of the ethanol extract of Magnolia officinalis and 4-O-methylhonokiol on scopolamine-induced memory impairment and the inhibition of acetylcholinesterase activity. J Nat Med. 2009; 63(3):274–282.11. Pachauri SD, Tota S, Khandelwal K, Verma PR, Nath C, Hanif K, Shukla R, Saxena JK, Dwivedi AK. Protective effect of fruits of Morinda citrifolia L. on scopolamine induced memory impairment in mice: a behavioral, biochemical and cerebral blood flow study. J Ethnopharmacol. 2012; 139(1):34–41.12. Tota S, Nath C, Najmi AK, Shukla R, Hanif K. Inhibition of central angiotensin converting enzyme ameliorates scopolamine induced memory impairment in mice: role of cholinergic neurotransmission, cerebral blood flow and brain energy metabolism. Behav Brain Res. 2012; 232(1):66–76.
Article13. Tanabe F, Miyasaka N, Kubota T, Aso T. Estrogen and progesterone improve scopolamine-induced impairment of spatial memory. J Med Dent Sci. 2004; 51(1):89–98.14. Adams B, Fitch T, Chaney S, Gerlai R. Altered performance characteristics in cognitive tasks: comparison of the albino ICR and CD1 mouse strains. Behav Brain Res. 2002; 133(2):351–361.
Article15. Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002; 136(2):489–501.
Article16. Brown RE, Wong AA. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn Mem. 2007; 14(3):134–144.
Article17. Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000; 7(3):170–179.
Article18. Buæan M, Abel T. The mouse: genetics meets behaviour. Nat Rev Genet. 2002; 3(2):114–123.
Article19. Song SH, Choi SM, Kim JE, Sung JE, Lee HA, Choi YH, Bae CJ, Choi YW, Hwang DY. α-Isocubebenol alleviates scopolamine-induced cognitive impairment by repressing acetylcholinesterase activity. Neurosci Lett. 2017; 638:121–128.
Article20. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11(1):47–60.
Article21. Kim MJ, Choi SJ, Lim ST, Kim HK, Kim YJ, Yoon HG, Shin DH. Zeatin supplement improves scopolamine-induced memory impairment in mice. Biosci Biotechnol Biochem. 2008; 72(2):577–581.
Article22. LeDoux JE. Emotional memory: in search of systems and synapses. Ann N Y Acad Sci. 1993; 702:149–157.
Article23. Prajapati KD, Sharma SS, Roy N. Upregulation of albumin expression in focal ischemic rat brain. Brain Res. 2010; 1327:118–124.
Article24. Ooigawa H, Nawashiro H, Fukui S, Otani N, Osumi A, Toyooka T, Shima K. The fate of Nissl-stained dark neurons following traumatic brain injury in rats: difference between neocortex and hippocampus regarding survival rate. Acta Neuropathol. 2006; 112(4):471–481.
Article25. Balaban H, Nazýroðlu M, Demirci K, Övey ÝS. The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: the involvement of TRPM2 and TRPV1 channels. Mol Neurobiol. 2017; 54(4):2852–2868.
Article26. Wong-Guerra M, Jiménez-Martin J, Pardo-Andreu GL, Fonseca-Fonseca LA, Souza DO, de Assis AM, Ramirez-Sanchez J, Del Valle RM, Nuñez-Figueredo Y. Mitochondrial involvement in memory impairment induced by scopolamine in rats. Neurol Res. 2017; 39(7):649–659.
Article27. Gella A, Durany N. Oxidative stress in Alzheimer disease. Cell Adh Migr. 2009; 3(1):88–93.
Article28. Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, Freedman ML. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer's disease. Exp Neurol. 1998; 150(1):40–44.
Article29. Perry G, Cash AD, Smith MA. Alzheimer disease and oxidative stress. J Biomed Biotechnol. 2002; 2(3):120–123.
Article30. Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer's disease. Biochim Biophys Acta. 2000; 1502(1):139–144.
Article31. Balu M, Sangeetha P, Haripriya D, Panneerselvam C. Rejuvenation of antioxidant system in central nervous system of aged rats by grape seed extract. Neurosci Lett. 2005; 383(3):295–300.
Article32. Mann H, McCoy MT, Subramaniam J, Van Remmen H, Cadet JL. Overexpression of superoxide dismutase and catalase in immortalized neural cells: toxic effects of hydrogen peroxide. Brain Res. 1997; 770(1-2):163–168.
Article33. Jain NK, Patil CS, Kulkarni SK, Singh A. Modulatory role of cyclooxygenase inhibitors in aging- and scopolamine or lipopolysaccharide-induced cognitive dysfunction in mice. Behav Brain Res. 2002; 133(2):369–376.
Article34. Lee B, Shim I, Lee H, Hahm DH. Rehmannia glutinosa ameliorates scopolamine-induced learning and memory impairment in rats. J Microbiol Biotechnol. 2011; 21(8):874–883.
Article35. Choi DY, Lee YJ, Lee SY, Lee YM, Lee HH, Choi IS, Oh KW, Han SB, Nam SY, Hong JT. Attenuation of scopolamine-induced cognitive dysfunction by obovatol. Arch Pharm Res. 2012; 35(7):1279–1286.
Article36. Liskowsky W, Schliebs R. Muscarinic acetylcholine receptor inhibition in transgenic Alzheimer-like Tg2576 mice by scopolamine favours the amyloidogenic route of processing of amyloid precursor protein. Int J Dev Neurosci. 2006; 24(2-3):149–156.
Article37. Gattu M, Boss KL, Terry AV Jr, Buccafusco JJ. Reversal of scopolamine-induced deficits in navigational memory performance by the seed oil of Celastrus paniculatus. Pharmacol Biochem Behav. 1997; 57(4):793–799.38. Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl). 1997; 132(2):107–124.
Article39. Yoneoka Y, Satoh M, Akiyama K, Sano K, Fujii Y, Tanaka R. An experimental study of radiation-induced cognitive dysfunction in an adult rat model. Br J Radiol. 1999; 72(864):1196–1201.
Article40. Beninger RJ, Jhamandas K, Boegman RJ, el-Defrawy SR. Effects of scopolamine and unilateral lesions of the basal forebrain on T-maze spatial discrimination and alternation in rats. Pharmacol Biochem Behav. 1986; 24(5):1353–1360.
Article41. Carballo-Márquez A, Vale-Martínez A, Guillazo-Blanch G, Torras-Garcia M, Boix-Trelis N, Martí-Nicolovius M. Differential effects of muscarinic receptor blockade in prelimbic cortex on acquisition and memory formation of an odor-reward task. Learn Mem. 2007; 14(9):616–624.42. Halder S, Mehta AK, Kar R, Mustafa M, Mediratta PK, Sharma KK. Clove oil reverses learning and memory deficits in scopolamine-treated mice. Planta Med. 2011; 77(8):830–834.
Article43. Chen KC, Baxter MG, Rodefer JS. Central blockade of muscarinic cholinergic receptors disrupts affective and attentional set-shifting. Eur J Neurosci. 2004; 20(4):1081–1088.
Article44. Liem-Moolenaar M, de Boer P, Timmers M, Schoemaker RC, van Hasselt JG, Schmidt S, van Gerven JM. Pharmacokinetic-pharmacodynamic relationships of central nervous system effects of scopolamine in healthy subjects. Br J Clin Pharmacol. 2011; 71(6):886–898.
Article45. Hosseini-Sharifabad A, Mohammadi-Eraghi S, Tabrizian K, Soodi M, Khorshidahmad T, Naghdi N, Abdollahi M, Beyer C, Roghani A, Sharifzadeh M. Effects of training in the Morris water maze on the spatial learning acquisition and VAChT expression in male rats. Daru. 2011; 19(2):166–172.46. Lamberty Y, Gower AJ. Cholinergic modulation of spatial learning in mice in a Morris-type water maze. Arch Int Pharmacodyn Ther. 1991; 309:5–19.47. Kim JS, Yang MY, Son YH, Kim SH, Kim JC, Kim SJ, Lee YD, Shin TK, Moon CJ. Strain-dependent differences of locomotor activity and hippocampus-dependent learning and memory in mice. Toxicol Res. 2008; 24(3):183–188.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Glycyrrhizic Acid on Scopolamine-Induced Cognitive Impairment in Mice
- A Novel Histone Deacetylase 6 Inhibitor, 4-FHA, Improves Scopolamine-Induced Cognitive and Memory Impairment in Mice
- Comparison of the virulence of Streptococcus pneumoniae in ICR mouse stocks of three different origins
- Cognitive Enhancing Activity of Lysimachia christinae Extract on Scopolamineinduced Memory Impairment in Mice
- Comparison of therapeutic responses to an anticancer drug in three stocks of ICR mice derived from three different sources