Yonsei Med J.
2019 Oct;60(10):924-934. 10.3349/ymj.2019.60.10.924.
microRNA-146a Promotes Growth of Acute Leukemia Cells by Downregulating Ciliary Neurotrophic Factor Receptor and Activating JAK2/STAT3 Signaling
- Affiliations
-
- 1Department of Pediatrics, Jinan Second Maternal and Child Health Hospital, Jinan, Shandong, China.
- 2Department of Pediatrics, Jinan First People's Hospital, Jinan, Shandong, China.
- 3Department of Pediatrics II, Yulin First Hospital, Suide, Shaanxi, China. leidonghong21@163.com
- KMID: 2459145
- DOI: http://doi.org/10.3349/ymj.2019.60.10.924
Abstract
- PURPOSE
Acute leukemia (AL) is classified as acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). This study aimed to investigate the effect of miR-146a on childhood AL and its underlying molecular mechanisms.
MATERIALS AND METHODS
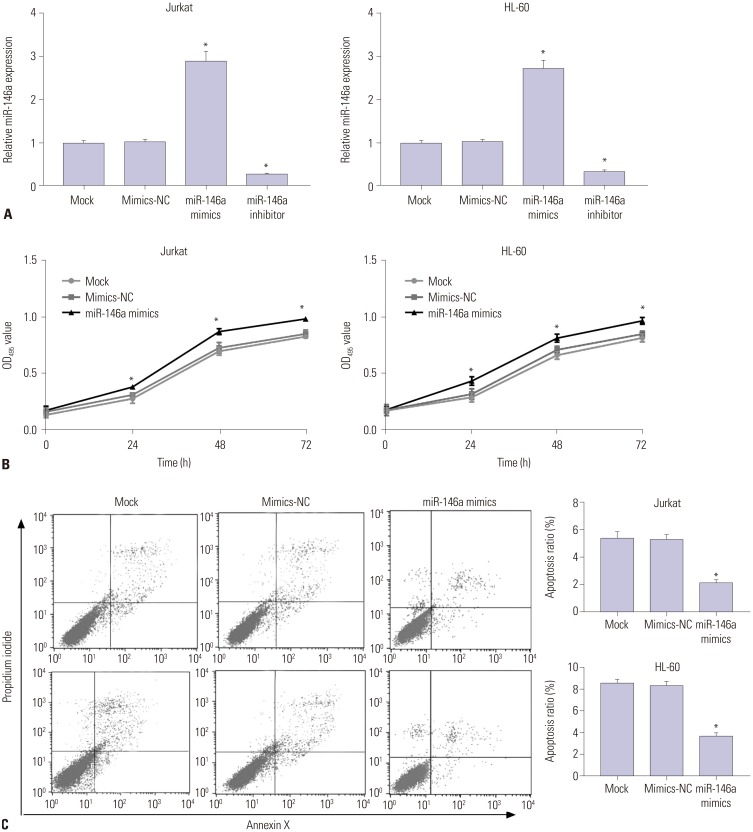
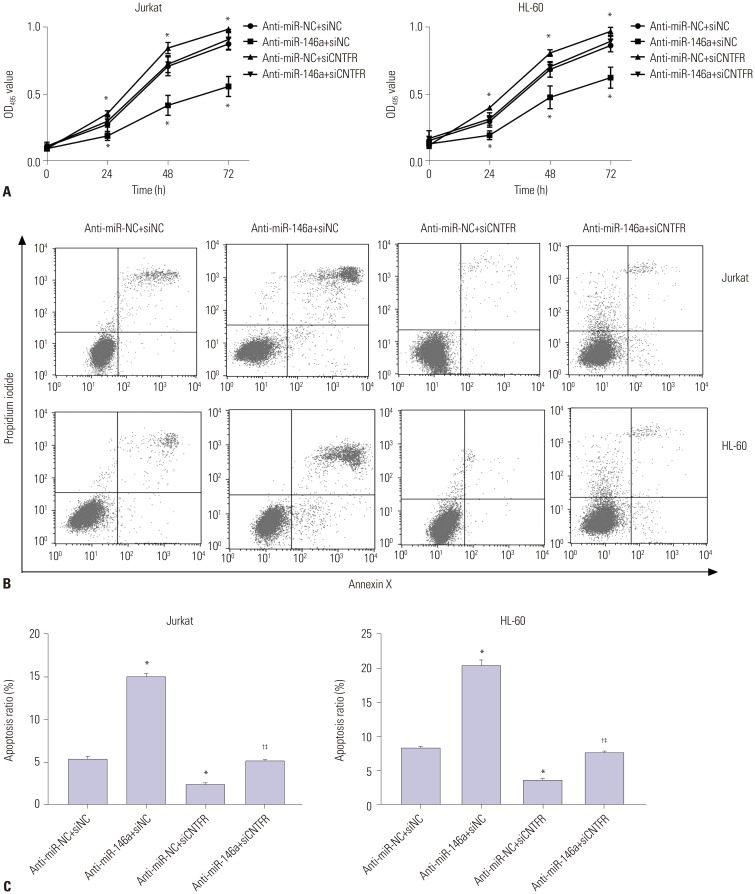
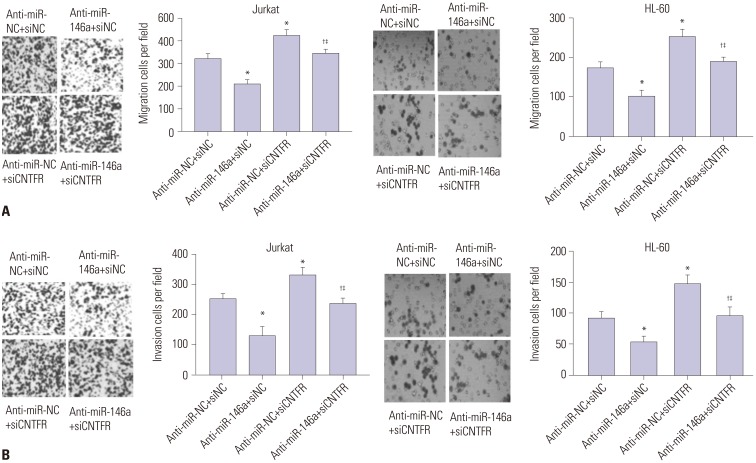
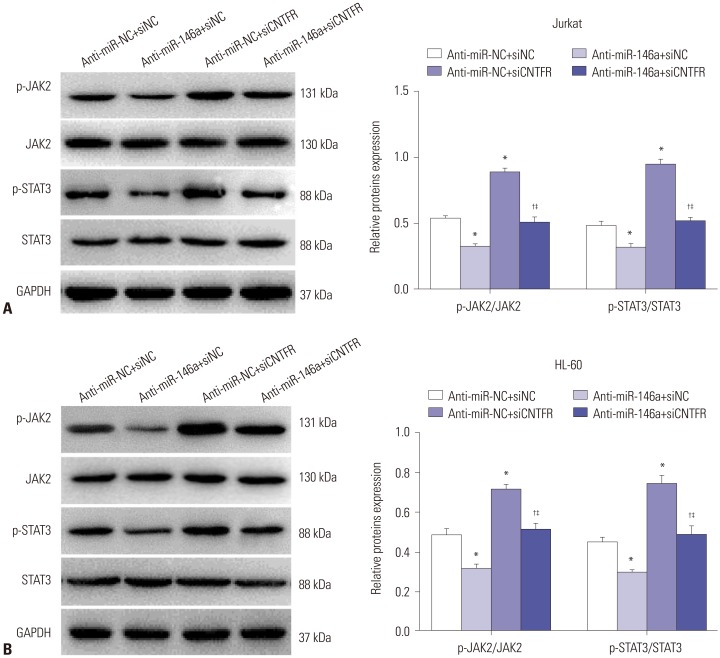
Bone marrow samples were obtained from 39 AL children and 10 non-cancer controls. The expressions of miR-146a and ciliary neurotrophic factor receptor (CNTFR) were detected by quantitative real-time polymerase chain reaction (qRT-PCR) in ALL and AML pediatric patients, as well as ALL (Jurkat) and AML (HL-60) cells. Correlations between miR-146a and clinical indicators were explored. A targeting relationship between miR-146a and CNTFR was detected by dual luciferase reporter gene assay. Cell proliferation, apoptosis, migration, and invasion of Jurkat and HL-60 cells were measured by MTT assay, flow cytometry, and transwell assay, respectively. LIF expression was detected by qRT-PCR in Jurkat and HL-60 cells. The expression of p-JAK2, JAK2, p-STAT3, and STAT3 in HL-60 cells was measured by Western blot.
RESULTS
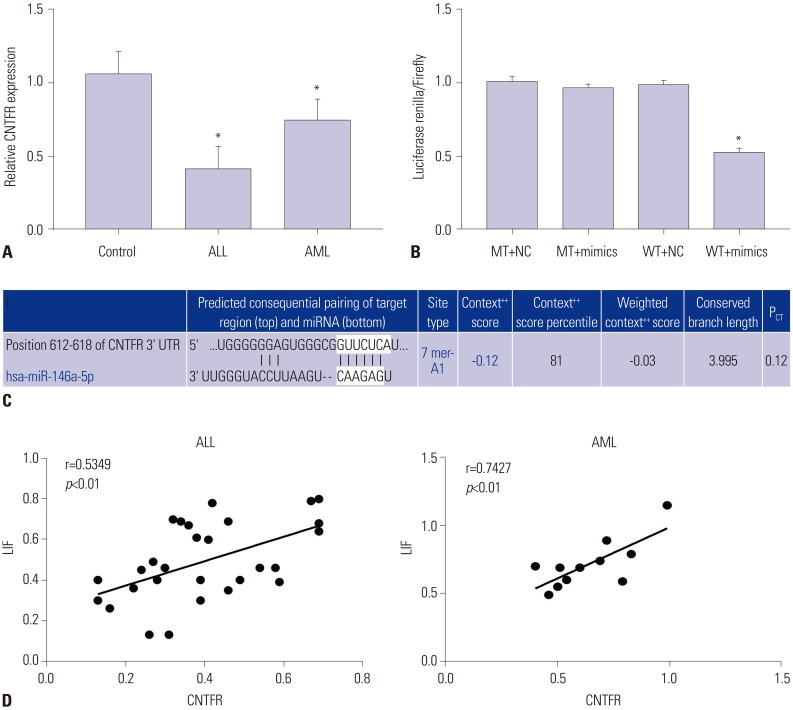
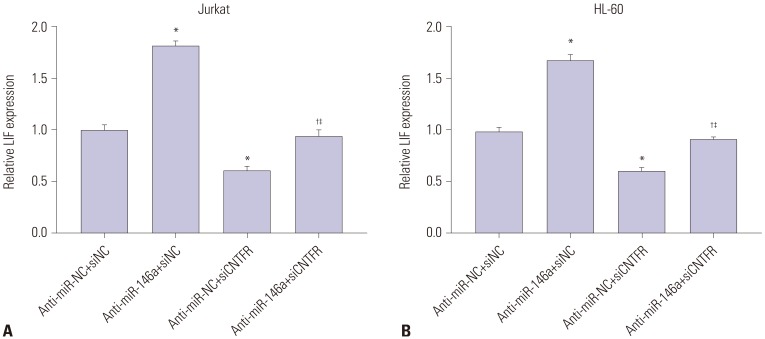
miR-146a was increased in ALL and AML pediatric patients, while CNTFR was decreased. miR-146a expression was associated with immunophenotype, karyotype, fusion gene, and SIL-TAL1. CNTFR was a target gene of miR-146a. miR-146a could promote cell proliferation, migration, and invasion, as well as inhibit cell apoptosis in Jurkat and HL-60 cells by downregulating CNTFR. Meanwhile, miR-146a inhibited the expression of LIF and activated JAK2/STAT3 pathway by downregulating CNTFR.
CONCLUSION
miR-146a could promote the proliferation, migration, and invasion and inhibit the apoptosis of AL Jurkat and HL-60 cells by downregulating CNTFR and activating the JAK2/STAT3 pathway.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Bone Marrow
Cell Proliferation
Child
Ciliary Neurotrophic Factor*
Flow Cytometry
Genes, Reporter
HL-60 Cells
Humans
Karyotype
Leukemia*
Leukemia, Myeloid, Acute
Luciferases
Precursor Cell Lymphoblastic Leukemia-Lymphoma
Real-Time Polymerase Chain Reaction
Receptor, Ciliary Neurotrophic Factor*
Ciliary Neurotrophic Factor
Luciferases
Receptor, Ciliary Neurotrophic Factor
Figure
Reference
-
1. Liu Z, Zhou T, Han X, Lang T, Liu S, Zhang P, et al. Mathematical models of amino acid panel for assisting diagnosis of children acute leukemia. J Transl Med. 2019; 17:38. PMID: 30674317.
Article2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. Ca Cancer J Clin. 2009; 59:225–249. PMID: 19474385.
Article3. Janitz AE, Campbell JE, Magzamen S, Pate A, Stoner JA, Peck JD. Traffic-related air pollution and childhood acute leukemia in Oklahoma. Environ Res. 2016; 148:102–111. PMID: 27038831.
Article5. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116:281–297. PMID: 14744438.6. Lin JF, Zeng H, Zhao JQ. MiR-212-5p regulates the proliferation and apoptosis of AML cells through targeting FZD5. Eur Rev Med Pharmacol Sci. 2018; 22:8415–8422. PMID: 30556883.7. Liu L, Ren W, Chen K. MiR-34a promotes apoptosis and inhibits autophagy by targeting HMGB1 in acute myeloid leukemia cells. Cell Physiol Biochem. 2017; 41:1981–1992. PMID: 28478444.
Article8. Huang K, Dong B, Wang Y, Tian T, Zhang B. MicroRNA-519 enhances HL60 human acute myeloid leukemia cell line proliferation by reducing the expression level of RNA-binding protein human antigen R. Mol Med Rep. 2015; 12:7830–7836. PMID: 26499919.
Article9. Hasani SS, Hashemi M, Eskandari-Nasab E, Naderi M, Omrani M, Sheybani-Nasab M. A functional polymorphism in the miR-146a gene is associated with the risk of childhood acute lymphoblastic leukemia: a preliminary report. Tumour Biol. 2014; 35:219–225. PMID: 23888320.
Article10. Hua Z, Chun W, Fang-Yuan C. MicroRNA-146a and hemopoietic disorders. Int J Hematol. 2011; 94:224–229. PMID: 21901398.
Article11. Starczynowski DT, Morin R, McPherson A, Lam J, Chari R, Wegrzyn J, et al. Genome-wide identification of human microRNAs located in leukemia-associated genomic alterations. Blood. 2011; 117:595–607. PMID: 20962326.
Article12. Ip NY, Nye SH, Boulton TG, Davis S, Taga T, Li Y, et al. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992; 69:1121–1132. PMID: 1617725.
Article13. Wagener EM, Aurich M, Aparicio-Siegmund S, Floss DM, Garbers C, Breusing K, et al. The amino acid exchange R28E in ciliary neurotrophic factor (CNTF) abrogates interleukin-6 receptor-dependent but retains CNTF receptor-dependent signaling via glycoprotein 130 (gp130)/leukemia inhibitory factor receptor (LIFR). J Biol Chem. 2014; 289:18442–18450. PMID: 24802752.
Article14. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011; 117:5019–5032. PMID: 21300984.
Article15. Liang X, Xin X, Qi D, Fu C, Ding M. Silencing the PIK3CA gene enhances the sensitivity of childhood leukemia cells to chemotherapy drugs by suppressing the phosphorylation of Akt. Yonsei Med J. 2019; 60:182–190. PMID: 30666840.16. Farah RA, Horkos JG, Bustros YD, Farhat HZ, Abla O. A multicenter experience from Lebanon in childhood and adolescent acute myeloid leukemia: high rate of early death in childhood acute promyelocytic leukemia. Mediterr J Hematol Infect Dis. 2015; 7:e2015012. PMID: 25574371.17. Yang R, Zhong L, Yang XQ, Jiang KL, Li L, Song H, et al. Neutrophil elastase enhances the proliferation and decreases apoptosis of leukemia cells via activation of PI3K/Akt signaling. Mol Med Rep. 2016; 13:4175–4182. PMID: 27035679.
Article18. Broglie L, Gershan J, Burke MJ. Checkpoint inhibition of PD-L1 and CTLA-4 in a child with refractory acute leukemia. Int J Hematol Oncol. 2019; 8:IJH10. PMID: 30863527.
Article19. Appelbaum FR, Kopecky KJ. Long-term survival after chemotherapy for acute myeloid leukemia: the experience of the Southwest Oncology Group. Cancer. 1997; 80(11 Suppl):2199–2204. PMID: 9395034.20. Boles JM, Dutel JL, Briere J, Mialon P, Robasckiewicz M, Garre M, et al. Acute renal failure caused by extreme hyperphosphatemia after chemotherapy of an acute lymphoblastic leukemia. Cancer. 1984; 53:2425–2429. PMID: 6585264.21. Agirre X, Martínez-Climent JÁ, Odero MD, Prósper F. Epigenetic regulation of miRNA genes in acute leukemia. Leukemia. 2012; 26:395–403. PMID: 22143672.
Article22. Wang Y, Tang P, Chen Y, Chen J, Ma R, Sun L. Overexpression of microRNA-125b inhibits human acute myeloid leukemia cells invasion, proliferation and promotes cells apoptosis by targeting NF-κB signaling pathway. Biochem Biophys Res Commun. 2017; 488:60–66. PMID: 28478034.
Article23. Zhang H, Luo XQ, Zhang P, Huang LB, Zheng YS, Wu J, et al. MicroRNA patterns associated with clinical prognostic parameters and CNS relapse prediction in pediatric acute leukemia. PLoS One. 2009; 4:e7826. PMID: 19915715.
Article24. Girard BM, Cheppudira BP, Malley SE, Schutz KC, May V, Vizzard MA. Increased expression of interleukin-6 family members and receptors in urinary bladder with cyclophosphamide-induced bladder inflammation in female rats. Front Neurosci. 2011; 5:20. PMID: 21373362.
Article25. Garbers C, Hermanns HM, Schaper F, Müller-Newen G, Grötzinger J, Rose-John S, et al. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012; 23:85–97. PMID: 22595692.
Article26. He W, Gong K, Zhu G, Smith DK, Ip NY. Membrane distal cytokine binding domain of LIFR interacts with soluble CNTFR in vitro. FEBS Lett. 2002; 514:214–218. PMID: 11943154.
Article27. Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev. 2015; 26:533–544. PMID: 26187859.
Article28. Onishi K, Zandstra PW. LIF signaling in stem cells and development. Development. 2015; 142:2230–2236. PMID: 26130754.
Article29. Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000; 19:5662–5679. PMID: 11114747.
Article30. Ward AC, Touw I, Yoshimura A. The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood. 2000; 95:19–29. PMID: 10607680.
Article31. Zhao J, Xu Y, Zong Y, Zhang S, Song Y, Yu K, et al. Inhibition of Stat3 expression induces apoptosis and suppresses proliferation in human leukemia HL-60 cells. Hematology. 2011; 16:232–235. PMID: 21756540.
Article32. Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999; 98:295–303. PMID: 10458605.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RAR-Related Orphan Receptor: An Accelerated Preeclampsia Progression by Activating the JAK/STAT3 Pathway
- Atorvastatin Pretreatment Ameliorates Mesenchymal Stem Cell Migration through miR-146a/CXCR4 Signaling
- Peroxisome Proliferator-activated Receptor-gamma Inhibits the Activation of STAT3 in Cerulein-stimulated Pancreatic Acinar Cells
- Expression of ciliary neurotrophic factor and its receptor in experimental obstructive nephropathy
- Hydroxyzine Induces Cell Death in Triple-Negative Breast Cancer Cells via Mitochondrial Superoxide and Modulation of Jak2/STAT3 Signaling