Obstet Gynecol Sci.
2019 Sep;62(5):322-328. 10.5468/ogs.2019.62.5.322.
Effects of genistein on anti-tumor activity of cisplatin in human cervical cancer cell lines
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, Chosun University, Gwangju, Korea. ksa@chosun.ac.kr
- 2Department of Internal Medicine, College of Medicine, Chosun University, Gwangju, Korea.
- KMID: 2456827
- DOI: http://doi.org/10.5468/ogs.2019.62.5.322
Abstract
OBJECTIVE
To investigate the effect of genistein on the anticancer effects of chemotherapeutic agents, we examined the effect of a genistein and cisplatin combination on CaSki human cervical cancer cells.
METHODS
After the cervical cancer cells (HeLa cells, CaSki cells) had been cultured, cisplatin and genistein were added to the culture medium, and the cell activity was measured using MTT assay. The CaSki cells were cultured in a medium containing cisplatin and genistein, and then, the cells were collected in order to measure p53, Bcl2, ERK, and caspase 3 levels by western blotting.
RESULTS
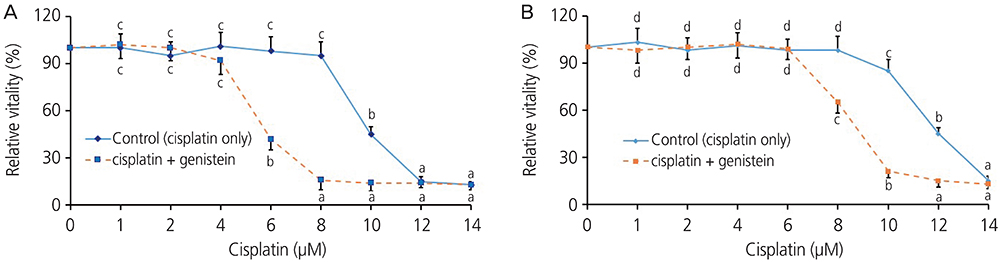
Both the HeLa and CaSki cells had decreased cell viabilities when the cisplatin concentration was 10 μM or higher. When combined with genistein, the cell viabilities of the HeLa and CaSki cells decreased at cisplatin concentrations of 8 μM and 6 μM, respectively. The administration of genistein increased the toxicity of cisplatin in the HeLa and CaSki cells. In the CaSki cells, the p-ERK1/2 level decreased by 37%, the p53 expression level increased by 304%, and the cleaved caspase 3 level increased by 115% in the cisplatin+genistein group compared to that in the cisplatin group. Bcl2 expression was reduced by 69% in the cisplatin+genistein group compared to that in the cisplatin group.
CONCLUSION
Genistein enhances the anticancer effect of cisplatin in CaSki cells, and can be used as a chemotherapeutic adjuvant to increase the activity of a chemotherapeutic agent.
Keyword
MeSH Terms
Figure
Reference
-
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Kumar L, Harish P, Malik PS, Khurana S. Chemotherapy and targeted therapy in the management of cervical cancer. Curr Probl Cancer. 2018; 42:120–128.
Article3. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014; 740:364–378.
Article4. Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005; 4:307–320.
Article5. Astolfi L, Ghiselli S, Guaran V, Chicca M, Simoni E, Olivetto E, et al. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: a retrospective evaluation. Oncol Rep. 2013; 29:1285–1292.
Article6. Matsuyama R, Reddy S, Smith TJ. Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol. 2006; 24:3490–3496.
Article7. Bartelink H, Schellens JH, Verheij M. The combined use of radiotherapy and chemotherapy in the treatment of solid tumours. Eur J Cancer. 2002; 38:216–222.
Article8. Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Anticancer efficacy of polyphenols and their combinations. Nutrients. 2016; 8:552–568.
Article9. Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008; 269:226–242.
Article10. Zhang Z, Wang CZ, Du GJ, Qi LW, Calway T, He TC, et al. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int J Oncol. 2013; 43:289–296.
Article11. Liu YL, Zhang GQ, Yang Y, Zhang CY, Fu RX, Yang YM. Genistein induces G2/M arrest in gastric cancer cells by increasing the tumor suppressor PTEN expression. Nutr Cancer. 2013; 65:1034–1041.
Article12. Yang YM, Yang Y, Dai WW, Li XM, Ma JQ, Tang LP. Genistein-induced apoptosis is mediated by endoplasmic reticulum stress in cervical cancer cells. Eur Rev Med Pharmacol Sci. 2016; 20:3292–3296.13. Tokalov SV, Abramyuk AM, Abolmaali ND. Protection of p53 wild type cells from taxol by genistein in the combined treatment of lung cancer. Nutr Cancer. 2010; 62:795–801.
Article14. Arzuman L, Beale P, Proschogo N, Yu JQ, Huq F. Combination of genistein and cisplatin with two designed monofunctional platinum agents in human ovarian tumour models. Anticancer Res. 2015; 35:6027–6039.15. Pons DG, Nadal-Serrano M, Torrens-Mas M, Oliver J, Roca P. The phytoestrogen genistein affects breast cancer cells treatment depending on the ERα/ERβ ratio. J Cell Biochem. 2016; 117:218–229.
Article16. Sahin K, Tuzcu M, Basak N, Caglayan B, Kilic U, Sahin F, et al. Sensitization of cervical cancer cells to cisplatin by genistein: the role of NFκB and Akt/mTOR signaling pathways. J Oncol. 2012; 2012:461562.
Article17. Sladowski D, Steer SJ, Clothier RH, Balls M. An improved MTT assay. J Immunol Methods. 1993; 157:203–207.18. Fulda S. Targeting apoptosis for anticancer therapy. Semin Cancer Biol. 2015; 31:84–88.
Article19. Kielbik M, Krzyzanowski D, Pawlik B, Klink M. Cisplatin-induced ERK1/2 activity promotes G1 to S phase progression which leads to chemoresistance of ovarian cancer cells. Oncotarget. 2018; 9:19847–19860.
Article20. Yang F, Guo L, Cao Y, Li S, Li J, Liu M. MicroRNA-7-5p promotes cisplatin resistance of cervical cancer cells and modulation of cellular energy homeostasis by regulating the expression of the PARP-1 and BCL2 genes. Med Sci Monit. 2018; 24:6506–6516.
Article21. Yang T, Xu F, Sheng Y, Zhang W, Chen Y. A targeted proteomics approach to the quantitative analysis of ERK/Bcl-2-mediated anti-apoptosis and multi-drug resistance in breast cancer. Anal Bioanal Chem. 2016; 408:7491–7503.
Article22. Wei F, Jiang X, Gao HY, Gao SH. Liquiritin induces apoptosis and autophagy in cisplatin (DDP)-resistant gastric cancer cells in vitro and xenograft nude mice in vivo. Int J Oncol. 2017; 51:1383–1394.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Glutathione on Cisplatin-Induced Cytotoxicity In Human Cervical Cancer Cell Lines
- The Change of Telomerase Activity by Cisplatin and 5-FU in Head and Neck Cancer Cell Lines
- ANTI-TUMOR EFFECT OF TAXOL AND CISPLATIN IN ORAL SQUAMOUS CELL CARCINOMA AND OSTEOSARCOMA CELL LINES
- The influence of p53 mutation status on the anti-cancer effect of cisplatin in oral squamous cell carcinoma cell lines
- Modification effects of recombinant human tumor necrosis factor and recombinant human interferon-gamma intrinsic and acquired resistance to cisplatin in human stomach and lung cancer cell lines