Ann Rehabil Med.
2019 Jun;43(3):328-334. 10.5535/arm.2019.43.3.328.
The Value of MicroRNAs as an Indicator of the Severity and the Acute Phase of Spinal Cord Injury
- Affiliations
-
- 1Department of Rehabilitation Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea. sadocean17@naver.com
- KMID: 2453923
- DOI: http://doi.org/10.5535/arm.2019.43.3.328
Abstract
OBJECTIVE
To assess the role of miRNA-21 and miRNA-223 in a balloon-compression model of spinal cord injury (SCI).
METHODS
A total of 50 male Wistar rats (n=50) were divided into the three groups: the group A (n=15, insertion of the unflated Fogarty balloon catheter), the group B (n=15, insertion of the Fogarty balloon catheter at a volume of 20 μL) and the group C (n=15, insertion of the Fogarty balloon catheter at a volume of 50 μL). After the behavioral test, RNA isolation, microRNA expression profiling using microarrays and quantitative polymerase chain reaction, measurements were compared between the three groups.
RESULTS
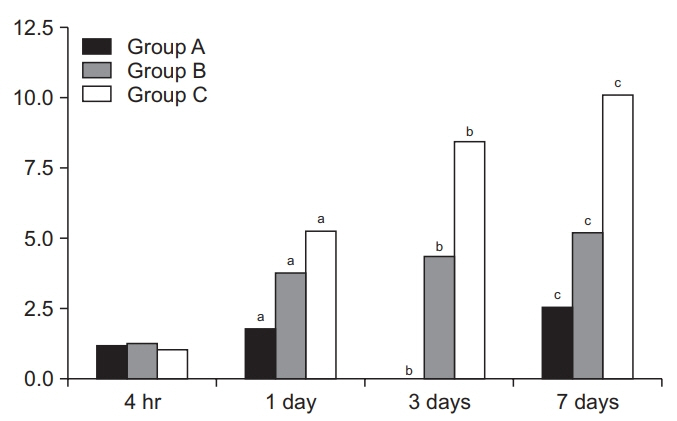
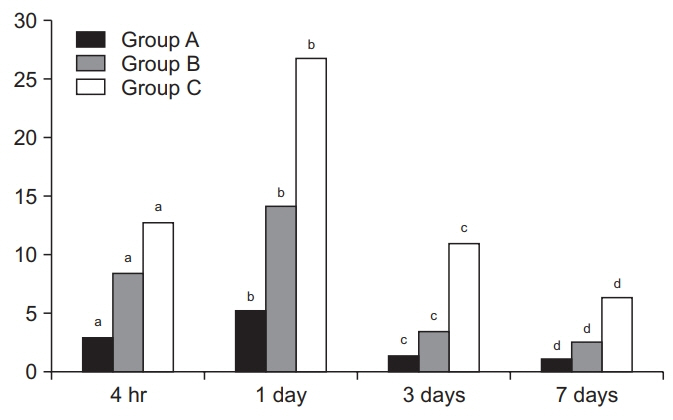
Despite a lack of significant differences in time-dependent changes in miRNA-21 expression levels between the three groups at 4 hours, there were significant differences in them at 1, 3, and 7 days (p<0.05). Moreover, there were significant differences in time-dependent changes in miRNA-223 expression levels between the three groups at 4 hours and 1, 3, and 7 days (p<0.05). Furthermore, miRNA-223 expression levels reached the highest at 1 day but were decreased with time thereafter in all the three groups.
CONCLUSION
Expression levels of miRNA-21 and miRNA-223 might be associated with the severity and acute phase of SCI, respectively. It is mandatory, however, to analyze changes in levels of inflammatory markers and the relevant biological pathways.
MeSH Terms
Figure
Reference
-
1. Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 2016; 10:98.
Article2. Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2012; 1:CD001046.
Article3. Budh CN, Osteraker AL. Life satisfaction in individuals with a spinal cord injury and pain. Clin Rehabil. 2007; 21:89–96.
Article4. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008; 25:E2.
Article5. Xu J, Kim GM, Chen S, Yan P, Ahmed SH, Ku G, et al. iNOS and nitrotyrosine expression after spinal cord injury. J Neurotrauma. 2001; 18:523–32.
Article6. Schaefer A, O’Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, et al. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007; 204:1553–8.
Article7. Carlson GD, Gorden C. Current developments in spinal cord injury research. Spine J. 2002; 2:116–28.
Article8. Silberstein M, Tress BM, Hennessy O. Prediction of neurologic outcome in acute spinal cord injury: the role of CT and MR. AJNR Am J Neuroradiol. 1992; 13:1597–608.9. Yilmaz T, Kaptanoglu E. Current and future medical therapeutic strategies for the functional repair of spinal cord injury. World J Orthop. 2015; 6:42–55.10. Hu JZ, Huang JH, Zeng L, Wang G, Cao M, Lu HB. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J Neurotrauma. 2013; 30:1349–60.
Article11. Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol. 2013; 9:328–39.
Article12. Hu JR, Lv GH, Yin BL. Altered microRNA expression in the ischemic-reperfusion spinal cord with atorvastatin therapy. J Pharmacol Sci. 2013; 121:343–6.13. Jee MK, Jung JS, Im YB, Jung SJ, Kang SK. Silencing of miR20a is crucial for Ngn1-mediated neuroprotection in injured spinal cord. Hum Gene Ther. 2012; 23:508–20.
Article14. Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol. 2009; 219:424–9.
Article15. Strickland ER, Hook MA, Balaraman S, Huie JR, Grau JW, Miranda RC. MicroRNA dysregulation following spinal cord contusion: implications for neural plasticity and repair. Neuroscience. 2011; 186:146–60.
Article16. Yunta M, Nieto-Diaz M, Esteban FJ, Caballero-Lopez M, Navarro-Ruiz R, Reigada D, et al. MicroRNA dysregulation in the spinal cord following traumatic injury. PLoS One. 2012; 7:e34534.
Article17. Zhou S, Ding F, Gu X. Non-coding RNAs as emerging regulators of neural injury responses and regeneration. Neurosci Bull. 2016; 32:253–64.
Article18. Yip PK, Malaspina A. Spinal cord trauma and the molecular point of no return. Mol Neurodegener. 2012; 7:6.
Article19. Izumi B, Nakasa T, Tanaka N, Nakanishi K, Kamei N, Yamamoto R, et al. MicroRNA-223 expression in neutrophils in the early phase of secondary damage after spinal cord injury. Neurosci Lett. 2011; 492:114–8.
Article20. Cheng X, Fu R, Gao M, Liu S, Li YQ, Song FH, et al. Intrathecal application of short interfering RNA knocks down c-jun expression and augments spinal motoneuron death after root avulsion in adult rats. Neuroscience. 2013; 241:268–79.
Article21. Zhou LH, Wu W. Survival of injured spinal motoneurons in adult rat upon treatment with glial cell linederived neurotrophic factor at 2 weeks but not at 4 weeks after root avulsion. J Neurotrauma. 2006; 23:920–7.
Article22. Vanicky I, Urdzikova L, Saganova K, Cizkova D, Galik J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma. 2001; 18:1399–407.23. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995; 12:1–21.
Article24. Barnabe-Heider F, Frisen J. Stem cells for spinal cord repair. Cell Stem Cell. 2008; 3:16–24.
Article25. Grulova I, Slovinska L, Nagyova M, Cizek M, Cizkova D. The effect of hypothermia on sensory-motor function and tissue sparing after spinal cord injury. Spine J. 2013; 13:1881–91.
Article26. Ulndreaj A, Badner A, Fehlings MG. Promising neuroprotective strategies for traumatic spinal cord injury with a focus on the differential effects among anatomical levels of injury. F1000Res. 2017; 6:1907.
Article27. Xiong Y, Mahmood A, Chopp M. Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs. 2009; 14:67–84.
Article28. Kjell J, Olson L. Rat models of spinal cord injury: from pathology to potential therapies. Dis Model Mech. 2016; 9:1125–37.
Article29. Lei P, Li Y, Chen X, Yang S, Zhang J. Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res. 2009; 1284:191–201.
Article30. Redell JB, Liu Y, Dash PK. Traumatic brain injury alters expression of hippocampal microRNAs: potential regulators of multiple pathophysiological processes. J Neurosci Res. 2009; 87:1435–48.
Article31. Kwon BK, Casha S, Hurlbert RJ, Yong VW. Inflammatory and structural biomarkers in acute traumatic spinal cord injury. Clin Chem Lab Med. 2011; 49:425–33.
Article32. Yokobori S, Zhang Z, Moghieb A, Mondello S, Gajavelli S, Dietrich WD, et al. Acute diagnostic biomarkers for spinal cord injury: review of the literature and preliminary research report. World Neurosurg. 2015; 83:867–78.
Article33. Kwon BK, Stammers AM, Belanger LM, Bernardo A, Chan D, Bishop CM, et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma. 2010; 27:669–82.
Article34. Lubieniecka JM, Streijger F, Lee JH, Stoynov N, Liu J, Mottus R, et al. Biomarkers for severity of spinal cord injury in the cerebrospinal fluid of rats. PLoS One. 2011; 6:e19247.
Article35. Dalkilic T, Fallah N, Noonan VK, Salimi Elizei S, Dong K, Belanger L, et al. Predicting injury severity and neurological recovery after acute cervical spinal cord injury: a comparison of cerebrospinal fluid and magnetic resonance imaging biomarkers. J Neurotrauma. 2018; 35:435–45.
Article36. Sengupta MB, Basu M, Iswarari S, Mukhopadhyay KK, Sardar KP, Acharyya B, et al. CSF proteomics of secondary phase spinal cord injury in human subjects: perturbed molecular pathways post injury. PLoS One. 2014; 9:e110885.
Article37. de la Torre JC. Spinal cord injury: review of basic and applied research. Spine (Phila Pa 1976). 1981; 6:315–35.38. Cheriyan T, Ryan DJ, Weinreb JH, Cheriyan J, Paul JC, Lafage V, et al. Spinal cord injury models: a review. Spinal Cord. 2014; 52:588–95.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anemia in the Acute Phase of Traumatic Spinal Cord Injury
- Acute Spinal Cord Injury after Cervical Nerve Root Block
- Recent Advances in the Pathophysiology and Treatment of Acute Spinal Cord Injury
- Dysesthetic Pain Syndrome in Spinal Cord Injury Patients
- The Differentiation of Phase of Spinal Cord Injury Based on the Changes in Gene Expression