J Korean Soc Spine Surg.
2011 Sep;18(3):75-82.
The Differentiation of Phase of Spinal Cord Injury Based on the Changes in Gene Expression
- Affiliations
-
- 1Department of Orthopedic Surgery, Chungnam National University, School of Medicine, Daejeon, Korea. jyyang@cnu.ac.kr
Abstract
-
STUDY DESIGN: An experimental study.
OBJECTIVES
To define the phases of chronic spinal cord injury by researching the changes in gene expression. SUMMARY OF LITERATURE REVIEW: The exact time of conversion from acute stage to chronic stage in spinal cord injury is unknown.
MATERIALS AND METHODS
We used 18 month-old Beagle dogs as study subjects. Under spinal cord monitoring, we underwent laminectomy on thoracic vertebra 10 and 11, and induced cord injury by a weight-drop injury method. Dogs in each group with spinal cord injury and group without spinal cord injury on POD 1, 7, 30, and 90. The motor functions were evaluated using the Tarlov scale. Tissues were prepared from 0.5cm up and down from the 10th thoracic level. Additional cephalic and caudal lesions from the injured site were prepared. We have checked the differentially expressed gene(DEG).
RESULTS
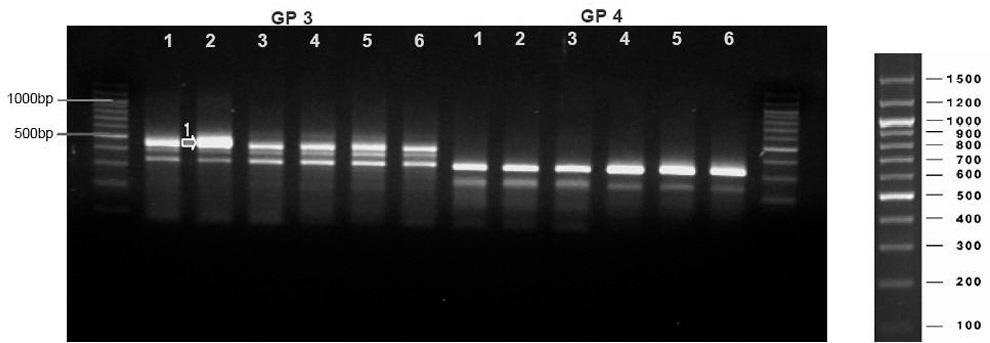
The mean Tarlov value was 0.67 which indicated a significant cord injury. 4 DEG (GP3, 9, 25, 34) were detected among 40 primers after screening, the detection percentage of which was 10. In the tissues of study subjects with spinal injury, DEG was found at the injury site and cephalic lesion. DEG expressed GP3, GP9 and GP34 started expression on day 30, and GP25 was expressed on day 90.
CONCLUSIONS
According to the changes in gene expression, the day 30 would be considered as the date of conversion from acute to chronic phase of cord injury. Inhibiting secondary inflammatory change and apoptosis following spinal cord injury until this period would maximize the effect of chronic phase therapy such as cell-transplantation.
MeSH Terms
Figure
Reference
-
1. Bunge RP, Puckett WR, Hiester ED. Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv Neurol. 1997; 72:305–15.2. Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004; 24:2143–55.
Article3. Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J comp Neurol. 2005; 486:373–83.
Article4. Balentine JD. Pathology of experimental spinal cord trauma. II. Ultrastructure of axons and myelin. Lab Invest. 1978; 39:254–66.5. Hofsteller C. Cell Therapy for Spinal Cord injury, Studies of Motor and Sensory Systems. Thesis. Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden. 2005.6. Finkelstein SD, Gillespie JA, Markowitz RS, Johnson DD, Black P. Experimental spinal cord injury: Qualitative and qualitative histopathologic evaluation. J Neurotrauma. 1990; 7:29–40.7. Bunge MB. Bridging areas of injury in the spinal cord. Neuroscientist. 2001; 7:325–39.8. Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980; 284:264–5.9. Yang JY, Lee JK, Rhee KJ, Kim KC, Hong UP, Lee JB. A Comparative Study of Behavioral and immunohistological Changes after Spinal Cord Injury between Young and Adult Rats. J Korean Orthop Assoc. 2004; 39:522–30.
Article10. Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001; 153:1133–40.
Article11. Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 1997; 3:73–6.12. Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999; 49:377–91.
Article13. Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004; 45:1–16.
Article14. Hong CH, Yang JY, Lee JK, Song HS. The Effect of Methylprednisolone and Riluzole on Axonal Growth after Acute Spinal Cord Injury in Rats. J Korean Orthop Assoc. 2008; 43:783–90.
Article15. Schreiber SL. Chemical genetics resulting from a passion for synthetic organic chemistry. Bioorg MedChem. 1998; 6:1127–52.
Article16. Bhaumik S, Lewis XZ, Gambhir SS. Optical imaging of Renilla luciferase, synthetic Renilla luciferase, and firefly luciferase reporter gene expression in living mice. J Biomed opt. 2004; 9:578–86.
Article17. Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002; 22:6623–30.
Article18. Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004; 21:754–74.
Article19. Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997; 14:67–116.
Article20. Ogawa Y, Sawamoto K, Miyata T, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002; 69:925–33.
Article21. Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006; 7:628–43.
Article22. Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000; 1:20–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Gene Expression in Mouse Spinal Cord-derived Neural Precursor Cells During Neuronal Differentiation
- The Value of MicroRNAs as an Indicator of the Severity and the Acute Phase of Spinal Cord Injury
- A Gene and Neural Stem Cell Therapy Platform Based on Neuronal Cell Type-Inducible Gene Overexpression
- The Time-course of Neurologic Recovery in Traumatic Spinal Cord Injury
- Identification and characterization of scirr1, a novel gene up-regulated after spinal cord injury