J Korean Neurosurg Soc.
2019 May;62(3):353-360. 10.3340/jkns.2019.0105.
A Primer on Magnetic Resonance-Guided Laser Interstitial Thermal Therapy for Medically Refractory Epilepsy
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea.
- 2Division of Neurosurgery, Department of Surgery, Toronto Western Hospital, University of Toronto, Toronto, Canada.
- 3Krembil Research Institute, University Health Network, Toronto, Canada.
- 4Department of Neurological Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. hongsound@gmail.com
- KMID: 2453241
- DOI: http://doi.org/10.3340/jkns.2019.0105
Abstract
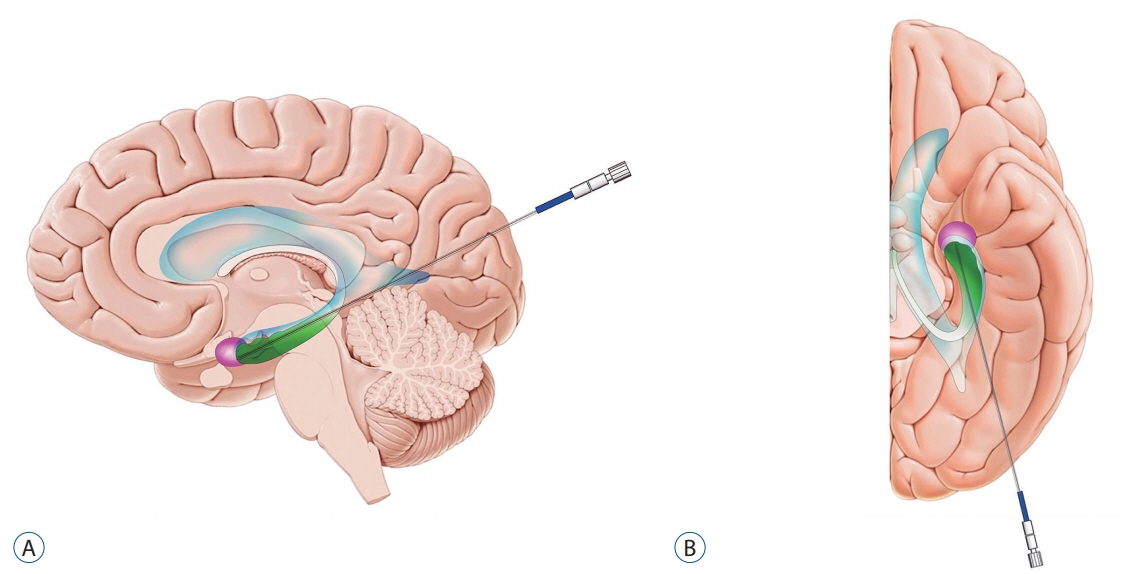
- Epilepsy surgery that eliminates the epileptogenic focus or disconnects the epileptic network has the potential to significantly improve seizure control in patients with medically intractable epilepsy. Magnetic resonance-guided laser interstitial thermal therapy (MRgLITT) has been an established option for epilepsy surgery since the US Food and Drug Administration cleared the use of MRgLITT in neurosurgery in 2007. MRgLITT is an ablative stereotactic procedure utilizing heat that is converted from laser energy, and the temperature of the tissue is monitored in real-time by MR thermography. Real-time quantitative thermal monitoring enables titration of laser energy for cellular injury, and it also estimates the extent of tissue damage. MRgLITT is applicable for lesion ablation in cases that the epileptogenic foci are localized and/or deep-seated such as in the mesial temporal lobe epilepsy and hypothalamic hamartoma. Seizure-free outcomes after MRgLITT are comparable to those of open surgery in well-selected patients such as those with mesial temporal sclerosis. Particularly in patients with hypothalamic hamartoma. In addition, MRgLITT can also be applied to ablate multiple discrete lesions of focal cortical dysplasia and tuberous sclerosis complex without the need for multiple craniotomies, as well as disconnection surgery such as corpus callosotomy. Careful planning of the target, the optimal trajectory of the laser probe, and the appropriate parameters for energy delivery are paramount to improve the seizure outcome and to reduce the complication caused by the thermal damage to the surrounding critical structures.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Clinical Applicability and Safety of Conventional Frame-Based Stereotactic Techniques for Stereoelectroencephalography
Junhyung Kim, Seok Ho Hong, Hyun-Jin Kim, Mi-Sun Yum, Tae Sung Ko, Yong Seo Koo, Sang-Ahm Lee
J Korean Neurosurg Soc. 2024;67(6):661-674. doi: 10.3340/jkns.2023.0246.
Reference
-
References
1. Abla AA, Rekate HL, Wilson DA, Wait SD, Uschold TD, Prenger E, et al. Orbitozygomatic resection for hypothalamic hamartoma and epilepsy: patient selection and outcome. Childs Nerv Syst. 27:265–277. 2011.
Article2. Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 40:445–452. 1999.
Article3. Bezchlibnyk YB, Willie JT, Gross RE. A neurosurgeon`s view: laser interstitial thermal therapy of mesial temporal lobe structures. Epilepsy Res. 142:135–139. 2018.
Article4. Chibbaro S, Cebula H, Scholly J, Todeschi J, Ollivier I, Timofeev A, et al. Pure endoscopic management of epileptogenic hypothalamic hamartomas. Neurosurg Rev. 40:647–653. 2017.
Article5. Curry DJ, Gowda A, McNichols RJ, Wilfong AA. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. 24:408–414. 2012.
Article6. Curry DJ, Raskin J, Ali I, Wilfong AA. MR-guided laser ablation for the treatment of hypothalamic hamartomas. Epilepsy Res. 142:131–134. 2018.
Article7. Drane DL. MRI-Guided stereotactic laser ablation for epilepsy surgery: promising preliminary results for cognitive outcome. Epilepsy Res. 142:170–175. 2018.
Article8. Drane DL, Loring DW, Voets NL, Price M, Ojemann JG, Willie JT, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 56:101–113. 2015.
Article9. Engel J Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 307:922–930. 2012.
Article10. Engel J Jr, Wiebe S, French J, Sperling M, Williamson P, Spencer D, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 60:538–547. 2003.
Article11. Esquenazi Y, Kalamangalam GP, Slater JD, Knowlton RC, Friedman E, Morris SA, et al. Stereotactic laser ablation of epileptogenic periventricular nodular heterotopia. Epilepsy Res. 108:547–554. 2014.
Article12. Fayed I, Sacino MF, Gaillard WD, Keating RF, Oluigbo CO. MR-guided laser interstitial thermal therapy for medically refractory lesional epilepsy in pediatric patients: experience and outcomes. Pediatr Neurosurg. 53:322–329. 2018.
Article13. Gross RE, Stern MA, Willie JT, Fasano RE, Saindane AM, Soares BP, et al. Stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Ann Neurol. 83:575–587. 2018.
Article14. Ho AL, Sussman ES, Pendharkar AV, Le S, Mantovani A, Keebaugh AC, et al. Improved operative efficiency using a real-time MRI-guided stereotactic platform for laser amygdalohippocampotomy. J Neurosurg. 128:1165–1172. 2018.
Article15. Hoppe C, Helmstaedter C, et al. Laser interstitial thermotherapy (LiTT) in pediatric epilepsy surgery. Seizure. 2018; [Epub ahead of print].
Article16. Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Coordination Active du Reseau Observatoire Longitudinal de l’ Epilepsie. Epilepsia. 42:464–475. 2001.
Article17. Jermakowicz WJ, Ivan ME, Cajigas I, Ribot R, Jusue-Torres I, Desai MB, et al. Visual deficit from laser interstitial thermal therapy for temporal lobe epilepsy: anatomical considerations. Oper Neurosurg (Hagerstown). 13:627–633. 2017.
Article18. Jermakowicz WJ, Kanner AM, Sur S, Bermudez C, D’Haese PF, Kolcun JPG, et al. Laser thermal ablation for mesiotemporal epilepsy: analysis of ablation volumes and trajectories. Epilepsia. 58:801–810. 2017.
Article19. Josephson CB, Dykeman J, Fiest KM, Liu X, Sadler RM, Jette N, et al. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology. 80:1669–1676. 2013.
Article20. Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 57:325–334. 2016.
Article21. Kerrigan JF, Ng YT, Chung S, Rekate HL. The hypothalamic hamartoma: a model of subcortical epileptogenesis and encephalopathy. Semin Pediatr Neurol. 12:119–131. 2005.
Article22. Kumar R, Yadav J, Sahu JK, Tripathi M, Ahuja C, Dayal D. Episodes of prolonged “trance-like state” in an infant with hypothalamic hamartoma. Ann Pediatr Endocrinol Metab. 24:55–59. 2019.
Article23. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 51:1069–1077. 2010.
Article24. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 342:314–319. 2000.
Article25. LaRiviere MJ, Gross RE. Stereotactic laser ablation for medically intractable epilepsy: the next generation of minimally invasive epilepsy surgery. Front Surg. 3:64. 2016.
Article26. Larson PS, Starr PA, Bates G, Tansey L, Richardson RM, Martin AJ. An optimized system for interventional magnetic resonance imaging-guided stereotactic surgery: preliminary evaluation of targeting accuracy. Neurosurgery. 70(1 Suppl Operative):95–103. discussion 103. 2012.
Article27. Le S, Ho AL, Fisher RS, Miller KJ, Henderson JM, Grant GA, et al. Laser interstitial thermal therapy (LITT): seizure outcomes for refractory mesial temporal lobe epilepsy. Epilepsy Behav. 89:37–41. 2018.
Article28. Lewis EC, Weil AG, Duchowny M, Bhatia S, Ragheb J, Miller I. MR-guided laser interstitial thermal therapy for pediatric drug-resistant lesional epilepsy. Epilepsia. 56:1590–1598. 2015.
Article29. Li K, Vakharia VN, Sparks R, Franca LGS, Granados A, McEvoy AW, et al. Optimizing trajectories for cranial laser interstitial thermal therapy using computer-assisted planning: a machine learning approach. Neurotherapeutics. 16:182–191. 2019.
Article30. Lutz MT, Clusmann H, Elger CE, Schramm J, Helmstaedter C. Neuropsychological outcome after selective amygdalohippocampectomy with transsylvian versus transcortical approach: a randomized prospective clinical trial of surgery for temporal lobe epilepsy. Epilepsia. 45:809–816. 2004.
Article31. Ng YT, Rekate HL, Prenger EC, Wang NC, Chung SS, Feiz-Erfan I, et al. Endoscopic resection of hypothalamic hamartomas for refractory symptomatic epilepsy. Neurology. 70:1543–1548. 2008.
Article32. North RY, Raskin JS, Curry DJ. MRI-guided laser interstitial thermal therapy for epilepsy. Neurosurg Clin N Am. 28:545–557. 2017.
Article33. Rekate HL, Feiz-Erfan I, Ng YT, Gonzalez LF, Kerrigan JF. Endoscopic surgery for hypothalamic hamartomas causing medically refractory gelastic epilepsy. Childs Nerv Syst. 22:874–880. 2006.
Article34. Shim KW, Park EK, Kim DS. Endoscopic treatment of hypothalamic hamartomas. J Korean Neurosurg Soc. 60:294–300. 2017.
Article35. Tovar-Spinoza Z, Carter D, Ferrone D, Eksioglu Y, Huckins S. The use of MRI-guided laser-induced thermal ablation for epilepsy. Childs Nerv Syst. 29:2089–2094. 2013.
Article36. Wiebe S, Blume WT, Girvin JP, Eliasziw M; Effectiveness, Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 345:311–318. 2001.
Article37. Wilfong AA, Curry DJ. Hypothalamic hamartomas: optimal approach to clinical evaluation and diagnosis. Epilepsia. 54 Suppl 9:109–114. 2013.
Article38. Willie JT, Laxpati NG, Drane DL, Gowda A, Appin C, Hao C, et al. Realtime magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 74:569–584. discussion 584-585. 2014.
Article39. Zolamorgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampalformation produce severe memory impairment. J Neurosci. 9:4355–4370. 1989.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to “A Primer on Magnetic Resonance-Guided Laser Interstitial Thermal Therapy for Medically Refractory Epilepsy†by Lee EJ, et al. (J Korean Neurosurg Soc 62: 353-360, 2019)
- Successful Use of Magnetic Resonance-Guided Focused Ultrasound Surgery for Long-Term Pain Palliation in a Patient Suffering from Metastatic Bone Tumor
- Clinical Manifestations and Surgical Outcome of Medically Refractory Epilepsy in Childhood
- Vagus Nerve Stimulation Therapy in Refractory Epilepsy: 18-Month Follow-up Multicenter Study
- Callosotomy for Intractable Epilepsy in Children