Yonsei Med J.
2019 Aug;60(8):751-759. 10.3349/ymj.2019.60.8.751.
Silencing of LncRNA-ANCR Promotes the Osteogenesis of Osteoblast Cells in Postmenopausal Osteoporosis via Targeting EZH2 and RUNX2
- Affiliations
-
- 1Department of Respiratory, Qingdao Eighth People's Hospital, Qingdao, Shandong, China.
- 2Department of Orthopedic Surgery, Qingdao University, Qingdao, Shandong, China.
- 3Department of Tramotology and Orthopedics, Pingyi Hospital of Traditional Chinese Medicine, Linyi, Shandong, China.
- 4Department of Sports Medicine, the First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China. wangfuke25@163.com
- KMID: 2452956
- DOI: http://doi.org/10.3349/ymj.2019.60.8.751
Abstract
- PURPOSE
This study aimed to explore the effects and mechanisms of long non-coding RNA (lncRNA) anti-differentiation non-coding RNA (ANCR) on the osteogenesis of osteoblast cells in postmenopausal osteoporosis (PMOP).
MATERIALS AND METHODS
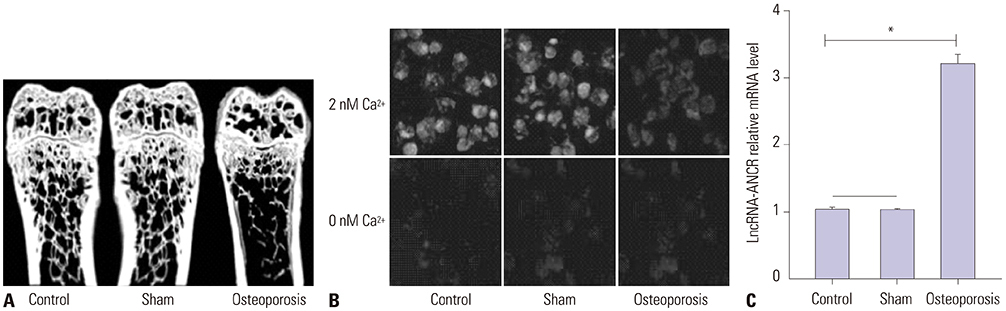
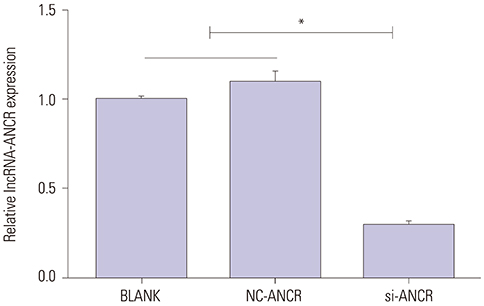
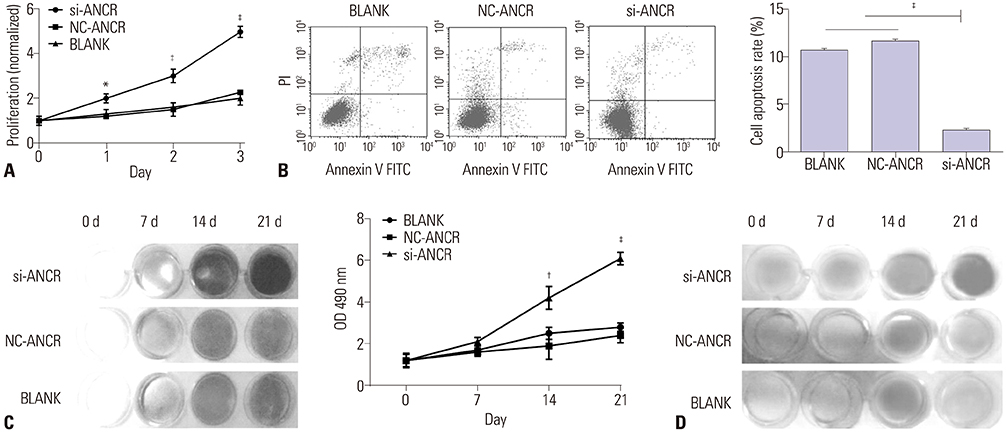
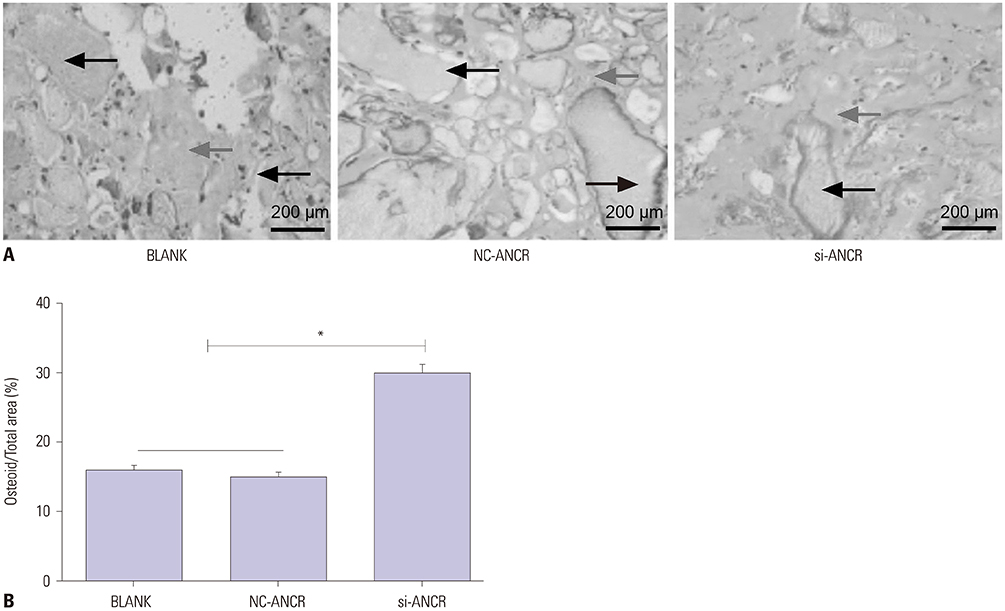
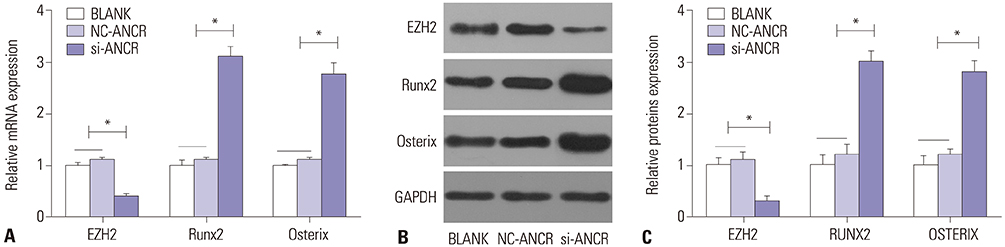
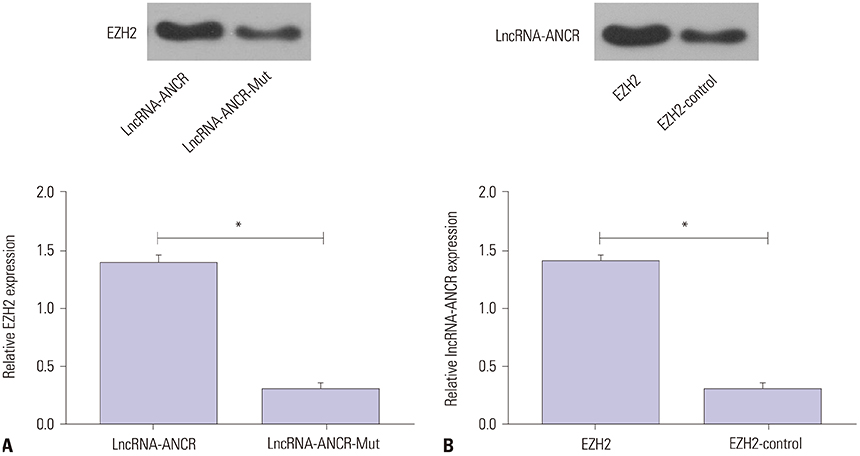
Mice models of PMOP were established. ANCR expression and intracellular calcium ions were detected by quantitative real-time polymerase chain reaction (qRT-PCR) and laser confocal microscopy, respectively. ANCR was silenced in osteoblast cells from PMOP mice by the transfection of siRNA-ANCR (si-ANCR). The proliferation and apoptosis of osteoblast cells was analyzed by MTT and flow cytometry, respectively. Alkaline phosphatase (ALP) activity and calcium nodules were examined by ALP and alizarin red staining assay, respectively. The expression of enhancer of zeste homolog 2 (EZH2), runt related transcription factor 2 (RUNX2), and OSTERIX was detected by qRT-PCR and Western blot. Furthermore, an osteogenesis model was constructed in mice, and osteoid formation was observed by hematoxylin-eosin (HE) staining. The interaction between lncRNA-ANCR and EZH2 was further identified by RNA pull-down assay.
RESULTS
ANCR expression and intracellular calcium ions were increased in PMOP mice. Si-ANCR significantly increased the proliferation, ALP activity, calcium deposition of osteoblast cells and decreased apoptosis. ANCR and EZH2 were down-regulated by si-ANCR, while RUNX2 and OSTERIX were upregulated. Si-ANCR also promoted osteoid formation in mice treated with hydroxyapatite-tricalcium phosphate. In addition, ANCR specifically bound to EZH2.
CONCLUSION
Silencing ANCR promotes the osteogenesis of PMOP osteoblast cells. The specific binding of ANCR with EZH2 suppressed RUNX2, thereby inhibiting osteogenesis.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Animals
Apoptosis
Blotting, Western
Calcium
Female
Flow Cytometry
Humans
Ions
Mice
Microscopy, Confocal
Osteoblasts*
Osteogenesis*
Osteoporosis, Postmenopausal*
Real-Time Polymerase Chain Reaction
RNA
RNA, Long Noncoding
RNA, Small Interfering
RNA, Untranslated
Transcription Factors
Transfection
Alkaline Phosphatase
Calcium
Ions
RNA
RNA, Long Noncoding
RNA, Small Interfering
RNA, Untranslated
Transcription Factors
Figure
Reference
-
1. Black DM, Rosen CJ. Postmenopausal osteoporosis. N Engl J Med. 2016; 374:2096–2097.
Article2. Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos. 2013; 8:137.
Article3. Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O'Malley CD. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos. 2014; 9:182.
Article4. Gennari L, Rotatori S, Bianciardi S, Nuti R, Merlotti D. Treatment needs and current options for postmenopausal osteoporosis. Expert Opin Pharmacother. 2016; 17:1141–1152.
Article5. Alami S, Hervouet L, Poiraudeau S, Briot K, Roux C. Barriers to effective postmenopausal osteoporosis treatment: a qualitative study of patients' and practitioners' views. Plos One. 2016; 11:e0158365.
Article6. Chang CY, Tang CH, Chen KC, Huang KC, Huang KC. The mortality and direct medical costs of osteoporotic fractures among postmenopausal women in Taiwan. Osteoporos Int. 2016; 27:665–676.
Article7. Baum R, Gravallese EM. Bone as a target organ in rheumatic disease: impact on osteoclasts and osteoblasts. Clin Rev Allergy Immunol. 2016; 51:1–15.
Article8. Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17-and TNF-α-induced genes in bone cells. J Leukoc Biol. 2005; 77:388–399.
Article9. Marie PJ, Kassem M. Osteoblasts in osteoporosis: past, emerging, and future anabolic targets. Eur J Endocrinol. 2011; 165:1–10.
Article10. Trošt Z, Trebše R, Preželj J, Komadina R, Logar DB, Marc J. A microarray based identification of osteoporosis-related genes in primary culture of human osteoblasts. Bone. 2010; 46:72–80.
Article11. Kretz M, Webster DE, Flockhart RJ, Lee CS, Zehnder A, Lopez-Pajares V, et al. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev. 2012; 26:338–343.
Article12. Jia Q, Jiang W, Ni L. Down-regulated non-coding RNA (lncRNA-ANCR) promotes osteogenic differentiation of periodontal ligament stem cells. Arch Oral Biol. 2015; 60:234–241.
Article13. Zhu L, Xu PC. Downregulated LncRNA-ANCR promotes osteoblast differentiation by targeting EZH2 and regulating Runx2 expression. Biochem Biophys Res Commun. 2013; 432:612–617.
Article14. Yang ZY, Yang F, Zhang YL, Liu B, Wang M, Hong X, et al. LncRNA-ANCR down-regulation suppresses invasion and migration of colorectal cancer cells by regulating EZH2 expression. Cancer Biomark. 2017; 18:95–104.
Article15. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001; 25:402–408.
Article16. Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003; 423:349–355.
Article17. Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005; 146:1226–1235.
Article18. Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005; 5:21–28.
Article19. Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: pathophysiology of osteoblast inhibition. Blood. 2006; 108:3992–3996.
Article20. Xiao X, Zhou T, Guo S, Guo C, Zhang Q, Dong N, et al. LncRNA MALAT1 sponges miR-204 to promote osteoblast differentiation of human aortic valve interstitial cells through up-regulating Smad4. Int J Cardiol. 2017; 243:404–412.
Article21. McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002; 3:737–747.
Article22. Li G, Zou L, Xie W, Wen S, Xie Q, Gao Y, et al. The effects of NONRATT021972 lncRNA siRNA on PC12 neuronal injury mediated by P2X 7 receptor after exposure to oxygen-glucose deprivation. Purinergic Signal. 2016; 12:479–487.
Article23. Shao Z, Zhang W, Fu R, Li L, Wang H. TET2 gene expression in bone marrow cells in myelodysplastic syndromes patients and the effect of silencing TET2 by siRNA on biological characteristics of healthy CD34+ cells. Blood. 2012; 120:4934.
Article24. Tan JZ, Yan Y, Wang XX, Jiang Y, Xu HE. EZH2: biology, disease, and structure-based drug discovery. Acta Pharmacol Sin. 2014; 35:161–174.
Article25. Ding X, Wang X, Sontag S, Qin J, Wanek P, Lin Q, et al. The polycomb protein Ezh2 impacts on induced pluripotent stem cell generation. Stem Cells Dev. 2014; 23:931–940.
Article26. Liu L, Luo Q, Sun J, Ju Y, Morita Y, Song G. Chromatin organization regulated by EZH2-mediated H3K27me3 is required for OPN-induced migration of bone marrow-derived mesenchymal stem cells. Int J Biochem Cell Biol. 2018; 96:29–39.
Article27. Chen YH, Chung CC, Liu YC, Yeh SP, Hsu JL, Hung MC, et al. Enhancer of zeste homolog 2 and histone deacetylase 9c regulate age-dependent mesenchymal stem cell differentiation into osteoblasts and adipocytes. Stem Cells. 2016; 34:2183–2193.
Article28. Dudakovic A, Camilleri ET, Paradise CR, Samsonraj RM, Gluscevic M, Paggi CA, et al. Enhancer of zeste homolog 2 (Ezh2) controls bone formation and cell cycle progression during osteogenesis in mice. J Biol Chem. 2018; 293:12894–12907.
Article29. Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ, et al. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget. 2015; 6:41045–41055.
Article30. Li Z, Hou P, Fan D, Dong M, Ma M, Li H, et al. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 2017; 24:59–71.
Article31. Zhang F, Peng H. LncRNA-ANCR regulates the cell growth of osteosarcoma by interacting with EZH2 and affecting the expression of p21 and p27. J Orthop Surg Res. 2017; 12:103.
Article32. Lucero CM, Vega OA, Osorio MM, Tapia JC, Antonelli M, Stein GS, et al. The cancer-related transcription factor Runx2 modulates cell proliferation in human osteosarcoma cell lines. J Cell Physiol. 2013; 228:714–723.
Article33. Fujita T, Azuma Y, Fukuyama R, Hattori Y, Yoshida C, Koida M, et al. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004; 166:85–95.
Article34. Yu C, Li L, Xie F, Guo S, Liu F, Dong N, et al. LncRNA TUG1 sponges miR-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification. Cardiovasc Res. 2018; 114:168–179.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LINC00662 Promotes Oral Squamous Cell Carcinoma Cell Growth and Metastasis through miR-144-3p/EZH2 Axis
- Remifentanil promotes osteoblastogenesis by upregulating Runx2/osterix expression in preosteoblastic C2C12 cells
- Aqueous extract of Petasites japonicus leaves promotes osteoblast differentiation via up-regulation of Runx2 and Osterix in MC3T3-E1 cells
- Function of runx2 and osterix in osteogenesis and teeth
- Regulation and Role of EZH2 in Cancer