Blood Res.
2018 Jun;53(2):152-159. 10.5045/br.2018.53.2.152.
The incidence of atypical patterns of BCR-ABL1 rearrangement and molecular-cytogenetic response to tyrosine kinase inhibitor therapy in newly diagnosed cases with chronic myeloid leukemia (CML)
- Affiliations
-
- 1Clinical Department of Laboratory Diagnostics, Division for Cytogenetics, University Hospital Centre Zagreb, Zagreb, Croatia. zeljka.tkalcic@gmail.com
- 2Department of Laboratory Diagnostics, General Hospital “Dr. Josip BenÄevićâ€, Slavonski Brod, Croatia.
- 3Clinical Department of Laboratory Diagnostics, Division of Laboratory Hematology and Coagulation, University Hospital Centre Zagreb, Zagreb, Croatia.
- 4Department of Medical Biochemistry and Hematology, University of Zagreb Faculty of Pharmacy and Biochemistry, Zagreb, Croatia.
- KMID: 2451034
- DOI: http://doi.org/10.5045/br.2018.53.2.152
Abstract
- BACKGROUND
To analyze the frequency of atypical fluorescence in situ hybridization signal patterns and estimate the complete cytogenetic response (CCyR) and major molecular response (MMR) during 12 months of tyrosine kinase inhibitor therapy in patients with newly diagnosed chronic myeloid leukemia.
METHODS
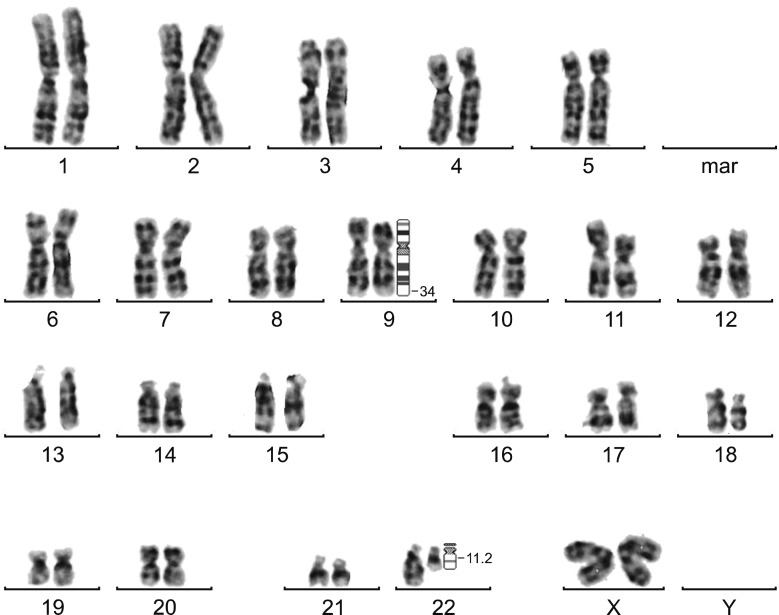
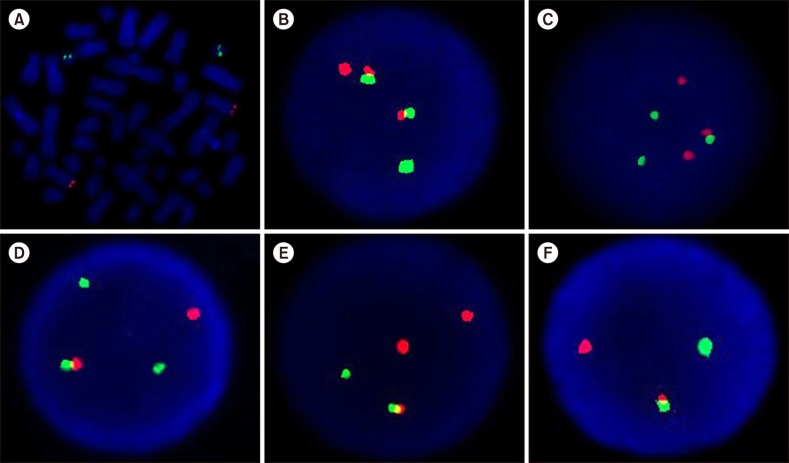
The study included bone marrow and peripheral blood samples from 122 patients with newly diagnosed chronic myeloid leukemia. Detection of the breakpoint cluster region"”Abelson fusion gene (BCR-ABL1) was performed using fluorescence in situ hybridization with a dual-color dual-fusion translocation probe, and MMR analysis was performed using the real-time quantitative polymerase chain reaction method.
RESULTS
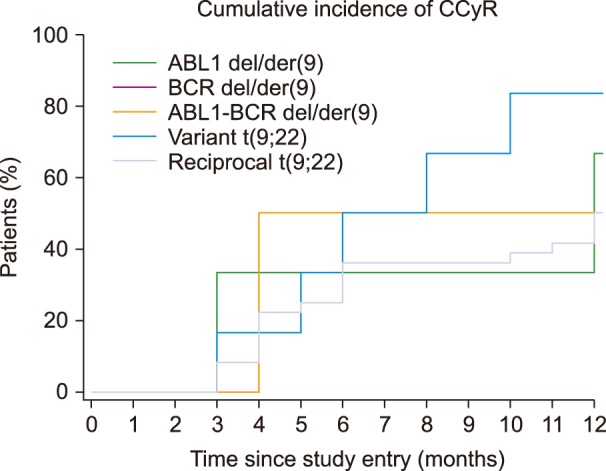
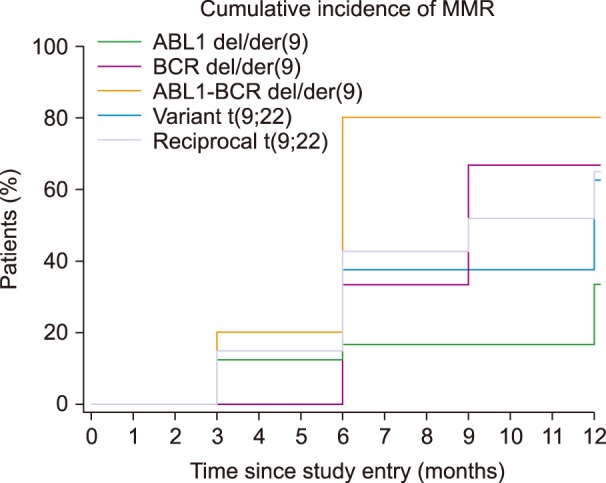
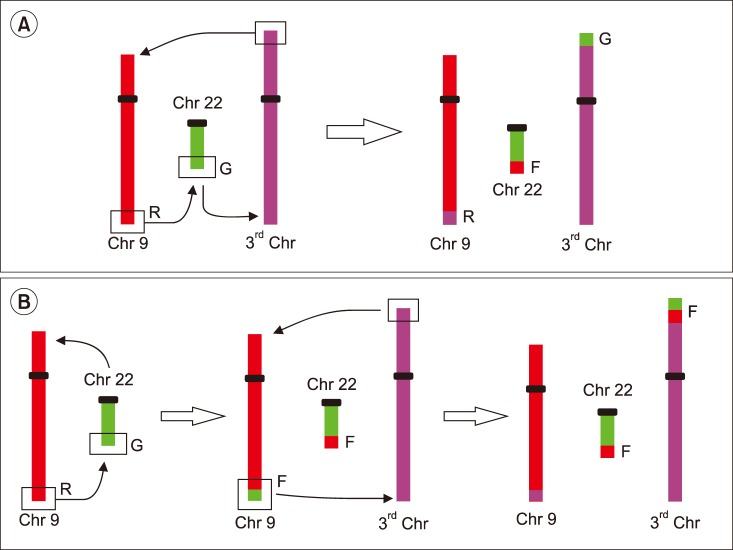
Variant translocation was determined in 10 samples and a deletion on the derivative chromosome 9 (del/der(9)) was found in 20 samples. The rates of CCyR and MMR were similar between patients with reciprocal translocation, variant translocation, deletion of derivative BCR, or ABL1-BCR fusion gene. The Kaplan-Meier test did not show any significant differences in the rates of CCyR and MMR among those groups of patients.
CONCLUSION
The frequencies of variant translocation and del/der(9) in the present study agree with the results of other studies performed worldwide. No differences were observed in the rates of CCyR and MMR between patients with atypical patterns and reciprocal translocation.
Keyword
MeSH Terms
Figure
Reference
-
1. Comert M, Baran Y, Saydam G. Changes in molecular biology of chronic myeloid leukemia in tyrosine kinase inhibitor era. Am J Blood Res. 2013; 3:191–200. PMID: 23997982.2. Suttorp M, Yaniv I, Schultz KR. Controversies in the treatment of CML in children and adolescents: TKIs versus BMT? Biol Blood Marrow Transplant. 2011; 17:S115–S122. PMID: 21195300.
Article3. Chomel JC, Brizard F, Veinstein A, et al. Persistence of BCR-ABL genomic rearrangement in chronic myeloid leukemia patients in complete and sustained cytogenetic remission after interferon-alpha therapy or allogeneic bone marrow transplantation. Blood. 2000; 95:404–408. PMID: 10627442.4. Salesse S, Verfaillie CM. BCR/ABL: from molecular mechanisms of leukemia induction to treatment of chronic myelogenous leukemia. Oncogene. 2002; 21:8547–8559. PMID: 12476301.
Article5. An X, Tiwari AK, Sun Y, Ding PR, Ashby CR Jr, Chen ZS. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk Res. 2010; 34:1255–1268. PMID: 20537386.
Article6. Greulich-Bode KM, Heinze B. On the power of additional and complex chromosomal aberrations in CML. Curr Genomics. 2012; 13:471–476. PMID: 23449041.7. Lim TH, Tien SL, Lim P, Lim AS. The incidence and patterns of BCR/ABL rearrangements in chronic myeloid leukaemia (CML) using fluorescence in situ hybridisation (FISH). Ann Acad Med Singapore. 2005; 34:533–538. PMID: 16284673.8. Rooney DE. Human cytogenetics: malignancy and acquired abnormalities. 3rd ed. Oxford, UK: Oxford University Press;2001.9. Shaffer LG, Slovak ML, Campbell LJ. ISCN 2009: An International System for Human Cytogenetic Nomenclature (2009). Basel, Switzerland: S. Karger;2009.10. Shaffer LG, McGowan JJ, Schmid M. ISCN 2013: An International System for Human Cytogenetic Nomenclature (2013). Basel, Switzerland: S. Karger;2013.11. Abbott Laboratories. Molecular oncology and genetics, product catalog 2016. Lake Bluff, IL: Abbott;2016. Accessed February 2, 2017. https://www.molecular.abbott/sal/en-us/staticAssets/AMD-US-Oncology-and-Genetics-Catalog.pdf.12. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013; 122:872–884. PMID: 23803709.13. Cross NC, White HE, Müller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012; 26:2172–2175. PMID: 22504141.
Article14. Cross NC, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015; 29:999–1003. PMID: 25652737.
Article15. Gadhia PK, Shastri GD, Shastri EG. Imatinib resistance and relapse in CML patients with complex chromosomal variants. Am J Cancer Sci. 2015; 4:43–53.16. Marzocchi G, Castagnetti F, Luatti S, et al. Variant Philadelphia translocations: molecular-cytogenetic characterization and prognostic influence on frontline imatinib therapy, a GIMEMA Working Party on CML analysis. Blood. 2011; 117:6793–6800. PMID: 21447834.
Article17. Sinclair PB, Nacheva EP, Leversha M, et al. Large deletions at the t(9;22) breakpoint are common and may identify a poor-prognosis subgroup of patients with chronic myeloid leukemia. Blood. 2000; 95:738–743. PMID: 10648381.
Article18. Huntly BJ, Reid AG, Bench AJ, et al. Deletions of the derivative chromosome 9 occur at the time of the Philadelphia translocation and provide a powerful and independent prognostic indicator in chronic myeloid leukemia. Blood. 2001; 98:1732–1738. PMID: 11535505.
Article19. Jain PP, Parihar M, Ahmed R, et al. Fluorescence in situ hybridization patterns of BCR/ABL1 fusion in chronic myelogenous leukemia at diagnosis. Indian J Pathol Microbiol. 2012; 55:347–351. PMID: 23032829.
Article20. Huntly BJ, Bench AJ, Delabesse E, et al. Derivative chromosome 9 deletions in chronic myeloid leukemia: poor prognosis is not associated with loss of ABL-BCR expression, elevated BCR-ABL levels, or karyotypic instability. Blood. 2002; 99:4547–4553. PMID: 12036887.21. Morel F, Ka C, Le Bris MJ, et al. Deletion of the 5′ABL region in Philadelphia chromosome positive chronic myeloid leukemia: frequency, origin and prognosis. Leuk Lymphoma. 2003; 44:1333–1338. PMID: 12952226.
Article22. Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006; 108:1809–1820. PMID: 16709930.
Article23. Huntly BJ, Bench A, Green AR. Double jeopardy from a single translocation: deletions of the derivative chromosome 9 in chronic myeloid leukemia. Blood. 2003; 102:1160–1168. PMID: 12730117.
Article24. Lee YK, Kim YR, Min HC, et al. Deletion of any part of the BCR or ABL gene on the derivative chromosome 9 is a poor prognostic marker in chronic myelogenous leukemia. Cancer Genet Cytogenet. 2006; 166:65–73. PMID: 16616113.
Article25. González FA, Anguita E, Mora A, et al. Deletion of BCR region 3' in chronic myelogenous leukemia. Cancer Genet Cytogenet. 2001; 130:68–74. PMID: 11672777.
Article26. Cohen N, Rozenfeld-Granot G, Hardan I, et al. Subgroup of patients with Philadelphia-positive chronic myelogenous leukemia characterized by a deletion of 9q proximal to ABL gene: expression profiling, resistance to interferon therapy, and poor prognosis. Cancer Genet Cytogenet. 2001; 128:114–119. PMID: 11463449.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Tyrosine Kinase Inhibitor in Chronic Myeloid Leukemia
- Asciminib: the first-in-class allosteric inhibitor of BCR::ABL1 kinase
- Isolated monocytosis was the flag preceding abnormalities in other parameters of complete blood counts in chronic myeloid leukemia with e1a2 (minor, P190) BCR-ABL1 chimeric transcripts
- Genomic Profiling of Chronic Myelogenous Leukemia: Basic and Clinical Approach
- A Case of Concomitant Inv(3)(q21q26) and Cryptic BCR/ABL1 Rearrangement in the Blast Crisis of Chronic Myeloid Leukemia