Tuberc Respir Dis.
2019 Jul;82(3):227-233. 10.4046/trd.2018.0070.
Clinical Characteristics of Korean Patients with Lung Cancer Who Have Programmed Death-Ligand 1 Expression
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. droij@jnu.ac.kr
- 2Lung and Esophageal Cancer Clinic, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3Department of Pathology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2450900
- DOI: http://doi.org/10.4046/trd.2018.0070
Abstract
- BACKGROUND
Programmed death-ligand 1 (PD-L1), a transmembrane protein, binds to the programmed death-1 (PD-1) receptor, and anti-PD-1 therapy enables immune responses against tumors. This study aimed to assess clinical characteristics of PD-L1 expression using immunohistochemistry among Korean patients with lung cancer.
METHODS
We retrospectively reviewed the data of patients with pathologically proven lung cancer from a single institution. PD-L1 expression determined by Tumor Proportion Score (TPS) was detected using 22C3 pharmDx (Agilent Technologies) and SP263 (Ventana Medical Systems) assays.
RESULTS
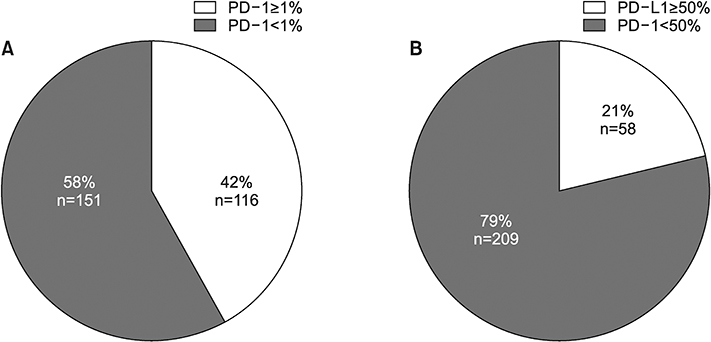
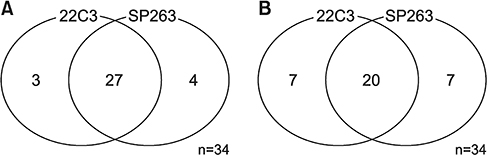
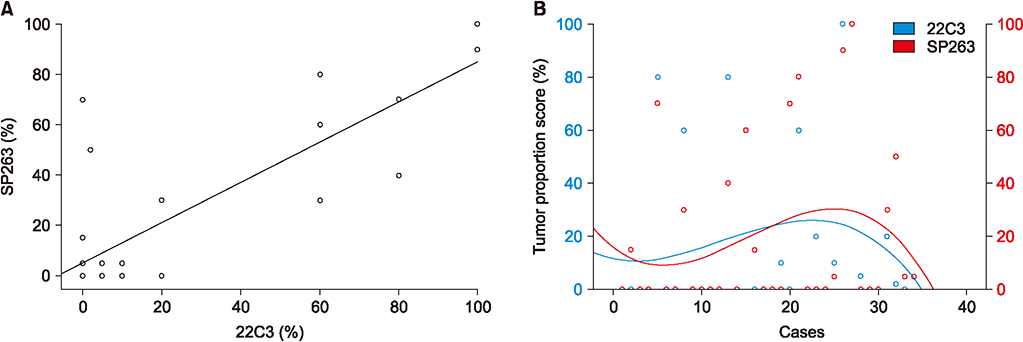
From July 2016 to July 2017, 267 patients were enrolled. The main histologic type was adenocarcinoma (69.3%). Most participants were smokers (67.4%) and had clinical stage IV disease (60.7%). In total, 116 (42%) and 58 (21%) patients had TPS ≥1% and ≥50%, respectively. The patients were significantly older in TPS ≥1% group than in TPS <1% group (64.83±9.38 years vs. 61.73±10.78 years, p=0.014), not in TPS ≥50% cutoff value (64.69 ± 9.39 vs. 62.36 ± 10.51, p= 0.178). Regarding histologic grade, higher proportions of poorly differentiated tumor were observed in the TPS ≥1% (40.8% vs. 25.8%, p=0.020) and TPS ≥50% groups (53.2% vs. 27.2%, p=0.004). Among 34 patients examined with 22C3 and SP263 assays, 27 had positive results in both assays, with a cutoff of TPS ≥1% (r=0.826; 95% confidence interval, 0.736-0.916).
CONCLUSION
PD-L1 expression, defined as TPS ??%, was related to older age and poorly differentiated histology. There was a similar distribution of PD-L1 expression in both 22C3 and SP263 results.
Keyword
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017; 67:7–30.
Article2. Park JY, Jang SH. Epidemiology of lung cancer in Korea: recent trends. Tuberc Respir Dis. 2016; 79:58–69.
Article3. Kweon SS. Updates on cancer epidemiology in Korea, 2018. Chonnam Med J. 2018; 54:90–100.
Article4. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–264.
Article5. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009; 206:3015–3029.6. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016; 375:1767–1778.
Article7. Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy: inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012; 18:6580–6587.8. Hall RD, Gray JE, Chiappori AA. Beyond the standard of care: a review of novel immunotherapy trials for the treatment of lung cancer. Cancer Control. 2013; 20:22–31.
Article9. Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction. J Immunother Cancer. 2016; 4:48.
Article10. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015; 14:847–856.
Article11. Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017; 12:208–222.
Article12. Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011; 28:682–688.
Article13. Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015; 89:181–188.
Article14. Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014; 25:1935–1940.
Article15. Roach C, Zhang N, Corigliano E, Jansson M, Toland G, Ponto G, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016; 24:392–397.
Article16. Rebelatto MC, Midha A, Mistry A, Sabalos C, Schechter N, Li X, et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol. 2016; 11:95.
Article17. Marchetti A, Barberis M, Franco R, De Luca G, Pace MV, Staibano S, et al. Multicenter comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) assays to test PD-L1 expression for NSCLC patients to be treated with immune check-point inhibitors. J Thorac Oncol. 2017; 12:1654–1663.
Article18. Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014; 94:107–116.
Article19. Scheel AH, Dietel M, Heukamp LC, Johrens K, Kirchner T, Reu S, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol. 2016; 29:1165–1172.
Article20. Adam J, Rouquette I, Damotte D, Badoual C, Danel C, Damiola F, et al. PL04a.04: multicentric French harmonization study for PD-L1 IHC testing in NSCLC. J Thorac Oncol. 2017; 12:1 Suppl. S11–S12.21. Ratcliffe MJ, Sharpe A, Midha A, Barker C, Scott M, Scorer P, et al. Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non-small cell lung cancer. Clin Cancer Res. 2017; 23:3585–3591.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An update on immunotherapy with PD-1 and PD-L1 blockade
- PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future
- Human Leukocyte Antigen Class I and Programmed Death-Ligand 1 Coexpression Is an Independent Poor Prognostic Factor in Adenocarcinoma of the Lung
- A Case of Anti-programmed Cell Death Ligand 1 and Anti-transforming Growth Factor Beta Antibody-associated Keratoacanthoma
- Immune Evasion of G-CSF and GM-CSF in Lung Cancer