J Pathol Transl Med.
2019 Jul;53(4):199-206. 10.4132/jptm.2019.04.24.
PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future
- Affiliations
-
- 1Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea. chungjh@snu.ac.kr
- 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2454598
- DOI: http://doi.org/10.4132/jptm.2019.04.24
Abstract
- Blockade of the programmed cell death-1 (PD-1) axis has already been established as an effective treatment of non-small cell lung cancer. Immunohistochemistry (IHC) for programmed death-ligand 1 (PD-L1) protein is the only available biomarker that can guide treatment with immune checkpoint inhibitors in non-small cell lung cancer. Because each PD-1/PD-L1 blockade was approved together with a specific PD-L1 IHC assay used in the clinical trials, pathologists have been challenged with performing various assays with a limited sample. To provide a more unified understanding of this, several cross-validation studies between platforms have been performed and showed consistent results. However, the interchangeability of assays may be limited in practice because of the risk of misclassification of patients for the treatment. Furthermore, several issues, including the temporal and spatial heterogeneity of PD-L1 expression in the tumor, and the potential for cytology specimens to be used as an alternative to tissue samples for PD-L1 testing, have still not been resolved. In the future, one of the main aims of immunotherapy research should be to find a novel predictive biomarker for PD-1 blockade therapy and a way to combine it with PD-L1 IHC and other tests.
Keyword
MeSH Terms
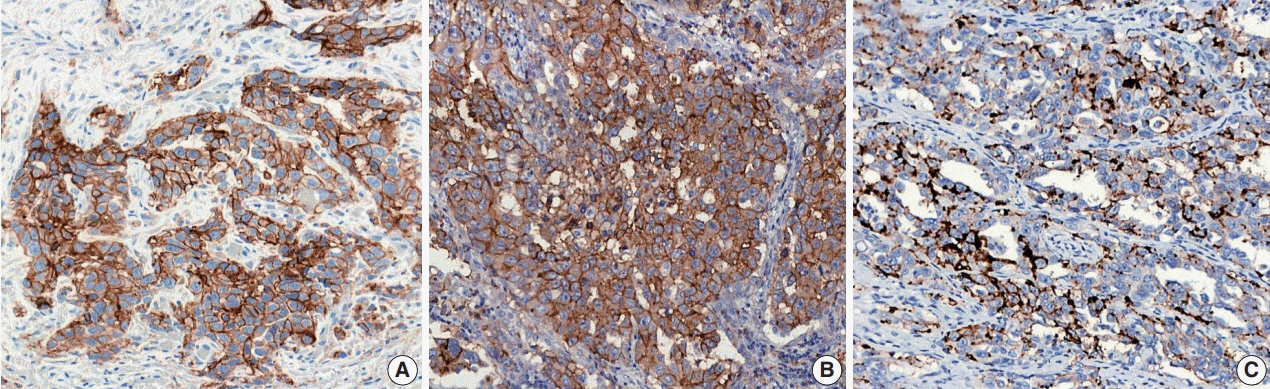
Figure
Cited by 1 articles
-
Current status and future perspectives of liquid biopsy in non-small cell lung cancer
Sunhee Chang, Jae Young Hur, Yoon-La Choi, Chang Hun Lee, Wan Seop Kim
J Pathol Transl Med. 2020;54(3):204-212. doi: 10.4132/jptm.2020.02.27.
Reference
-
1. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–39.
Article2. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–35.
Article3. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016; 375:1823–33.
Article4. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–50.
Article5. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016; 387:1837–46.
Article6. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017; 389:255–65.
Article7. Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017; 18:599–610.
Article8. Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018; 19:521–36.9. Non-small cell lung cancer. Version 3.2018 [Internet]. Plymouth Meeting: National Comprehensive Cancer Network;2018. [cited 2019 Mar 29]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.10. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002; 3:991–8.
Article11. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–64.
Article12. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011; 331:1565–70.
Article13. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012; 366:2455–65.
Article14. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017; 12:208–22.
Article15. Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol. 2016; 29:1165–72.
Article16. Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017; 3:1051–8.
Article17. Adam J, Le Stang N, Rouquette I, et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol. 2018; 29:953–8.
Article18. Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non-small cell lung cancer. Clin Cancer Res. 2017; 23:3585–91.
Article19. Sughayer MA, Alnaimy F, Alsughayer AM, Qamhia N. Comparison of 22C3 PharmDx and SP263 assays to test PD-L1 expression in NSCLC. Appl Immunohistochem Mol Morphol. 2018; Jul. 17. [Epub]. https://doi.org/10.1097/PAI.0000000000000671.
Article20. Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of Blueprint Phase 2 Project. J Thorac Oncol. 2018; 13:1302–11.
Article21. Fujimoto D, Sato Y, Uehara K, et al. Predictive performance of four programmed cell death ligand 1 assay systems on nivolumab response in previously treated patients with non-small cell lung cancer. J Thorac Oncol. 2018; 13:377–86.22. Marchetti A, Barberis M, Franco R, et al. Multicenter comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) assays to test PD-L1 expression for NSCLC patients to be treated with immune checkpoint inhibitors. J Thorac Oncol. 2017; 12:1654–63.
Article23. VENTANA PD-L1 (SP263) Assay (CE IVD) [Internet]. Indianapolis: Roche Diagnostics;2019. [cited 2019 Mar 29]. Available from: https://diagnostics.roche.com/global/en/products/tests/ventanapd-l1-_sp263-assay2.html.24. Munari E, Rossi G, Zamboni G, et al. PD-L1 assays 22C3 and SP263 are not interchangeable in non-small cell lung cancer when considering clinically relevant cutoffs: an interclone evaluation by differently trained pathologists. Am J Surg Pathol. 2018; 42:1384–9.25. Hendry S, Byrne DJ, Wright GM, et al. Comparison of four PD-L1 immunohistochemical assays in lung cancer. J Thorac Oncol. 2018; 13:367–76.
Article26. Tsao MS, Kerr KM, Dacic SA, Yatabe YA, Hirsch FR. IASLC atlas of PD-L1 immunohistochemistry testing in lung cancer. Aurora: International Association for the Study of Lung Cancer;2017.27. Herbst RS, Baas P, Perez-Gracia JL, et al. Use of archival versus newly collected tumor samples for assessing PD-L1 expression and overall survival: an updated analysis of KEYNOTE-010 trial. Ann Oncol. 2019; 30:281–9.
Article28. Omori S, Kenmotsu H, Abe M, et al. Changes in programmed death ligand 1 expression in non-small cell lung cancer patients who received anticancer treatments. Int J Clin Oncol. 2018; 23:1052–9.
Article29. Sheng J, Fang W, Yu J, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in nonsmall cell lung cancer. Sci Rep. 2016; 6:20090.
Article30. Shin J, Chung JH, Kim SH, et al. Effect of platinum-based chemotherapy on PD-L1 expression on tumor cells in non-small cell lung cancer. Cancer Res Treat. 2018; Nov. 5. [Epub]. https://doi.org/10.4143/crt.2018.537.
Article31. Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014; 25:1935–40.
Article32. Han JJ, Kim DW, Koh J, et al. Change in PD-L1 expression after acquiring resistance to gefitinib in EGFR-mutant non-small-cell lung cancer. Clin Lung Cancer. 2016; 17:263–70. e2.
Article33. Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PDL1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016; 27:147–53.
Article34. Gniadek TJ, Li QK, Tully E, Chatterjee S, Nimmagadda S, Gabrielson E. Heterogeneous expression of PD-L1 in pulmonary squamous cell carcinoma and adenocarcinoma: implications for assessment by small biopsy. Mod Pathol. 2017; 30:530–8.
Article35. Kim H, Kwon HJ, Park SY, Park E, Chung JH. PD-L1 immunohistochemical assays for assessment of therapeutic strategies involving immune checkpoint inhibitors in non-small cell lung cancer: a comparative study. Oncotarget. 2017; 8:98524–32.
Article36. Mansfield AS, Murphy SJ, Peikert T, et al. Heterogeneity of programmed cell death ligand 1 expression in multifocal lung cancer. Clin Cancer Res. 2016; 22:2177–82.
Article37. Kim S, Koh J, Kwon D, et al. Comparative analysis of PD-L1 expression between primary and metastatic pulmonary adenocarcinomas. Eur J Cancer. 2017; 75:141–9.
Article38. Niemeijer AN, Leung D, Huisman MC, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with nonsmall-cell lung cancer. Nat Commun. 2018; 9:4664.
Article39. Ilié M, Szafer-Glusman E, Hofman V, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol. 2018; 29:193–9.
Article40. Guibert N, Delaunay M, Lusque A, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer. 2018; 120:108–12.
Article41. Rebelatto MC, Midha A, Mistry A, et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol. 2016; 11:95.
Article42. Skov BG, Skov T. Paired comparison of PD-L1 expression on cytologic and histologic specimens from malignancies in the lung assessed with PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol. 2017; 25:453–9.
Article43. Heymann JJ, Bulman WA, Swinarski D, et al. PD-L1 expression in non-small cell lung carcinoma: comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol. 2017; 125:896–907.
Article44. Wang H, Agulnik J, Kasymjanova G, et al. Cytology cell blocks are suitable for immunohistochemical testing for PD-L1 in lung cancer. Ann Oncol. 2018; 29:1417–22.
Article45. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015; 348:124–8.46. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018; 378:2093–104.
Article47. Mazzaschi G, Madeddu D, Falco A, et al. Low PD-1 expression in cytotoxic CD8(+) tumor-infiltrating lymphocytes confers an immuneprivileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin Cancer Res. 2018; 24:407–19.
Article48. Kim H, Kwon HJ, Han YB, et al. Increased CD3+ T cells with a low FOXP3+/CD8+ T cell ratio can predict anti-PD-1 therapeutic response in non-small cell lung cancer patients. Mod Pathol. 2019; 32:367–75.
Article49. Postow MA, Manuel M, Wong P, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer. 2015; 3:23.
Article50. Weber JS, Sznol M, Sullivan RJ, et al. A serum protein signature associated with outcome after anti-PD-1 therapy in metastatic melanoma. Cancer Immunol Res. 2018; 6:79–86.
Article51. Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015; 350:1084–9.52. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014; 371:2189–99.
Article53. Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018; 33:853–61. e4.
Article54. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 372:2509–20.
Article55. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017; 376:2415–26.56. Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018; 36:633–41.
Article57. Deans ZC, Costa JL, Cree I, et al. Integration of next-generation sequencing in clinical diagnostic molecular pathology laboratories for analysis of solid tumours; an expert opinion on behalf of IQN Path ASBL. Virchows Arch. 2017; 470:5–20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PD-L1 as a Biomarker in Gastric Cancer Immunotherapy
- Correction of acknowledgments: PD-L1 testing in non-small cell lung cancer: past, present, and future
- Programmed death-ligand 1 expression and tumor-infiltrating lymphocytes in non-small cell lung cancer: association with clinicopathologic parameters
- Correlation of PD-L1 Expression Tested by 22C3 and SP263 in Non-Small Cell Lung Cancer and Its Prognostic Effect on EGFR Mutation–Positive Lung Adenocarcinoma
- Temporal evolution of programmed death-ligand 1 expression in patients with non-small cell lung cancer