Yonsei Med J.
2019 Jul;60(7):667-678. 10.3349/ymj.2019.60.7.667.
Poorly-Controlled Type 1 Diabetes Mellitus Impairs LH-LHCGR Signaling in the Ovaries and Decreases Female Fertility in Mice
- Affiliations
-
- 1Department of Biomedical Laboratory Science, College of Health Science, Eulji University, Seongnam, Korea.
- 2Department of Obstetrics and Gynecology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA. tkw@northwestern.edu
- 3Olson Center for Women's Health, Department of Obstetrics and Gynecology, and Fred and Pamela Buffett Cancer Center, University of Nebraska Medical Center, Omaha, NE, USA.
- 4Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea.
- KMID: 2450407
- DOI: http://doi.org/10.3349/ymj.2019.60.7.667
Abstract
- PURPOSE
The aim of this study was to investigate how type I diabetes mellitus (T1D) affects the folliculogenesis and oocyte development, fertilization, and embryo development.
MATERIALS AND METHODS
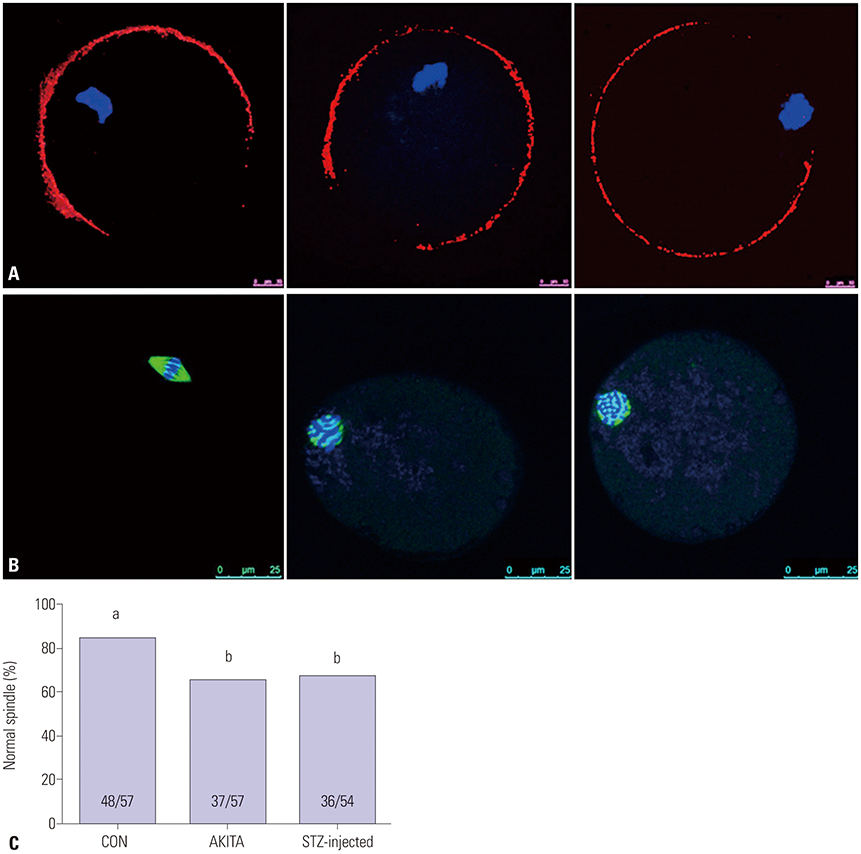
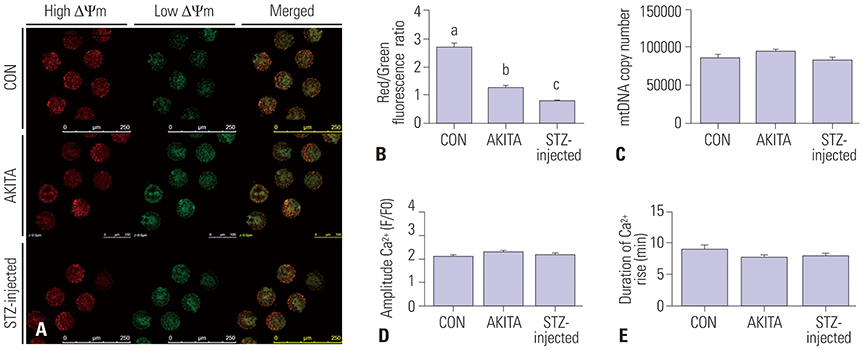
A comparative animal study was conducted using two different mouse models of T1D, a genetic AKITA model and a streptozotocin-induced diabetes model. Ovarian function was assessed by gross observation, immunoblot, immunohistochemistry, oocyte counting, and ELISA for serum hormones (insulin, anti-Mullerian hormone, estradiol, testosterone, and progesterone). Maturation and developmental competence of metaphase II oocytes from control and T1D animals was evaluated by immunofluorescent and immunohistochemical detection of biomarkers and in vitro fertilization.
RESULTS
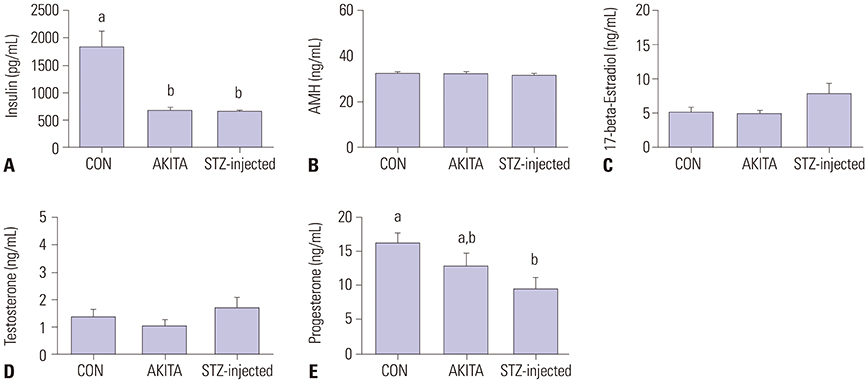
Animals from both T1D models showed increased blood glucose levels, while only streptozotocin (STZ)-injected mice showed reduced body weight. Folliculogenesis, oogenesis, and preimplantation embryogenesis were impaired in both T1D mouse models. Interestingly, exogenous streptozotocin injection to induce T1D led to marked decreases in ovary size, expression of luteinizing hormone/chorionic gonadotropin receptor in the ovaries, the number of corpora lutea per ovary, oocyte maturation, and serum progesterone levels. Both T1D models exhibited significantly reduced pre-implantation embryo quality compared with controls. There was no significant difference in embryo quality between STZ-injected and AKITA diabetic mice.
CONCLUSION
These results suggest that T1D affects folliculogenesis, oogenesis, and embryo development in mice. However, the physiological mechanisms underlying the observed reproductive effects of diabetes need to be further investigated.
MeSH Terms
-
Animals
Anti-Mullerian Hormone
Biomarkers
Blood Glucose
Body Weight
Corpus Luteum
Diabetes Mellitus
Diabetes Mellitus, Type 1*
Embryonic Development
Embryonic Structures
Enzyme-Linked Immunosorbent Assay
Estradiol
Female
Female*
Fertility*
Fertilization
Fertilization in Vitro
Gonadotropins
Humans
Immunohistochemistry
Lutein
Mental Competency
Metaphase
Mice*
Oocytes
Oogenesis
Ovary*
Pregnancy
Progesterone
Reproduction
Streptozocin
Testosterone
Anti-Mullerian Hormone
Biomarkers
Blood Glucose
Estradiol
Gonadotropins
Lutein
Progesterone
Streptozocin
Testosterone
Figure
Reference
-
1. Codner E, Merino PM, Tena-Sempere M. Female reproduction and type 1 diabetes: from mechanisms to clinical findings. Hum Reprod Update. 2012; 18:568–585.
Article2. Clark M, Kroger CJ, Tisch RM. Type 1 diabetes: a chronic anti-self-inflammatory response. Front Immunol. 2017; 8:1898.
Article3. Gerard-Gonzalez A, Gitelman SE, Cheng P, Dubose SN, Miller KM, Olson BA, et al. Comparison of autoantibody-positive and autoantibody-negative pediatric participants enrolled in the T1D Exchange clinic registry. J Diabetes. 2013; 5:216–223.
Article4. Abell SK, Nankervis A, Khan KS, Teede HJ. Type 1 and Type 2 diabetes preconception and in pregnancy: health impacts, influence of obesity and lifestyle, and principles of management. Semin Reprod Med. 2016; 34:110–120.
Article5. Codner E, Eyzaguirre FC, Iñiguez G, López P, Pérez-Bravo F, Torrealba IM, et al. Ovulation rate in adolescents with type 1 diabetes mellitus. Fertil Steril. 2011; 95:197–202.
Article6. Chang AS, Dale AN, Moley KH. Maternal diabetes adversely affects preovulatory oocyte maturation, development, and granulosa cell apoptosis. Endocrinology. 2005; 146:2445–2453.
Article7. Diamond MP, Moley KH, Pellicer A, Vaughn WK, DeCherney AH. Effects of streptozotocin- and alloxan-induced diabetes mellitus on mouse follicular and early embryo development. J Reprod Fertil. 1989; 86:1–10.
Article8. Ratchford AM, Chang AS, Chi MM, Sheridan R, Moley KH. Maternal diabetes adversely affects AMP-activated protein kinase activity and cellular metabolism in murine oocytes. Am J Physiol Endocrinol Metab. 2007; 293:E1198–E1206.
Article9. Wang Q, Frolova AI, Purcell S, Adastra K, Schoeller E, Chi MM, et al. Mitochondrial dysfunction and apoptosis in cumulus cells of type I diabetic mice. PLoS One. 2010; 5:e15901.
Article10. Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest. 1999; 103:27–37.
Article11. Yamane S, Hamamoto Y, Harashima S, Harada N, Hamasaki A, Toyoda K, et al. GLP-1 receptor agonist attenuates endoplasmic reticulum stress-mediated β-cell damage in Akita mice. J Diabetes Investig. 2011; 2:104–110.
Article12. Jo H, Byun HM, Lee SI, Shin DM. Initiation site of Ca(2+) entry evoked by endoplasmic reticulum Ca(2+) depletion in mouse parotid and pancreatic acinar cells. Yonsei Med J. 2007; 48:526–530.
Article13. Lee KG, Yoo MS, Choi IJ. The effect of corticosteroid on the fetal pulmonary maturation of rats with streptozotocin-induced diabetes. Yonsei Med J. 1986; 27:121–131.
Article14. Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, et al. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011; 45:131–140.
Article15. Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009; 103:378–386.
Article16. Choi YS, Song JE, Kong BS, Hong JW, Novelli S, Lee EJ. The role of Foxo3 in Leydig cells. Yonsei Med J. 2015; 56:1590–1596.
Article17. Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005; 46:2210–2218.
Article18. Kim SY, Ebbert K, Cordeiro MH, Romero M, Zhu J, Serna VA, et al. Cell autonomous phosphoinositide 3-kinase activation in oocytes disrupts normal ovarian function through promoting survival and overgrowth of ovarian follicles. Endocrinology. 2015; 156:1464–1476.
Article19. Kim SE, Koo JS, Jung WH. Immunophenotypes of glycogen rich clear cell carcinoma. Yonsei Med J. 2012; 53:1142–1146.
Article20. Kim EJ, Lee J, Youm HW, Kim SK, Lee JR, Suh CS, et al. Comparison of follicle isolation methods for mouse ovarian follicle culture in vitro. Reprod Sci. 2018; 25:1270–1278.
Article21. Mainigi MA, Ord T, Schultz RM. Meiotic and developmental competence in mice are compromised following follicle development in vitro using an alginate-based culture system. Biol Reprod. 2011; 85:269–276.
Article22. Richani D, Sutton-McDowall ML, Frank LA, Gilchrist RB, Thompson JG. Effect of epidermal growth factor-like peptides on the metabolism of in vitro- matured mouse oocytes and cumulus cells. Biol Reprod. 2014; 90:49.
Article23. Wiebe JC, Santana A, Medina-Rodríguez N, Hernández M, Nóvoa J, Mauricio D, et al. Fertility is reduced in women and in men with type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium (T1DGC). Diabetologia. 2014; 57:2501–2504.
Article24. Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC, et al. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development. 2015; 142:681–691.
Article25. Gordo AC, Kurokawa M, Wu H, Fissore RA. Modifications of the Ca2+ release mechanisms of mouse oocytes by fertilization and by sperm factor. Mol Hum Reprod. 2002; 8:619–629.
Article26. Sutton-McDowall ML, Wu LL, Purdey M, Abell AD, Goldys EM, MacMillan KL, et al. Nonesterified fatty acid-induced endoplasmic reticulum stress in cattle cumulus oocyte complexes alters cell metabolism and developmental competence. Biol Reprod. 2016; 94:23.
Article27. Zhang N, Duncan FE, Que EL, O'Halloran TV, Woodruff TK. The fertilization-induced zinc spark is a novel biomarker of mouse embryo quality and early development. Sci Rep. 2016; 6:22772.
Article28. Lee J, Kim J, Kim SH, Kang HG, Jun JH. Effects of coculture with immune cells on the developmental competence of mouse preimplantation embryos in vitro and in utero. Reprod Sci. 2015; 22:1252–1261.
Article29. Chillarón JJ, Benaiges D, Mañé L, Pedro-Botet J, Flores Le-Roux JA. Obesity and type 1 diabetes mellitus management. Minerva Endocrinol. 2015; 40:53–60.30. Schoeller EL, Albanna G, Frolova AI, Moley KH. Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes. 2012; 61:1869–1878.
Article31. Schoeller EL, Chi M, Drury A, Bertschinger A, Esakky P, Moley KH. Leptin monotherapy rescues spermatogenesis in male Akita type 1 diabetic mice. Endocrinology. 2014; 155:2781–2786.
Article32. Johnson LM, Sidman RL. A reproductive endocrine profile in the diabetes (db) mutant mouse. Biol Reprod. 1979; 20:552–559.33. Wang Q, Chi MM, Moley KH. Live imaging reveals the link between decreased glucose uptake in ovarian cumulus cells and impaired oocyte quality in female diabetic mice. Endocrinology. 2012; 153:1984–1989.
Article34. Wang Q, Moley KH. Maternal diabetes and oocyte quality. Mitochondrion. 2010; 10:403–410.
Article35. Burkart AD, Xiong B, Baibakov B, Jiménez-Movilla M, Dean J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J Cell Biol. 2012; 197:37–44.
Article36. Xiong B, Zhao Y, Beall S, Sadusky AB, Dean J. A unique egg cortical granule localization motif is required for ovastacin sequestration to prevent premature ZP2 cleavage and ensure female fertility in mice. PLoS Genet. 2017; 13:e1006580.
Article37. Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010; 83:52–62.38. Zhang CH, Qian WP, Qi ST, Ge ZJ, Min LJ, Zhu XL, et al. Maternal diabetes causes abnormal dynamic changes of endoplasmic reticulum during mouse oocyte maturation and early embryo development. Reprod Biol Endocrinol. 2013; 11:31.
Article39. Moley KH, Chi MM, Mueckler MM. Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am J Physiol. 1998; 275:E38–E47.40. Chi MM, Hoehn A, Moley KH. Metabolic changes in the glucose-induced apoptotic blastocyst suggest alterations in mitochondrial physiology. Am J Physiol Endocrinol Metab. 2002; 283:E226–E232.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glycogenic hepatopathy in a Korean girl with poorly controlled type 1 diabetes mellitus
- Self-Management and Its Predictors for Patients with Poorly Controlled Type 2 Diabetes
- Low Serum Testosterone Concentrations in Hospitalized Men with Poorly Controlled Type 2 Diabetes
- Ovarian Development of Vitrified Neonatal Ovaries after Orthotopic Transplantation into Adult Recipients
- Blood Pressure Target in Type 2 Diabetes Mellitus