Yonsei Med J.
2015 May;56(3):760-771. 10.3349/ymj.2015.56.3.760.
Stimulating Effect of a Novel Synthesized Sulfonamido-Based Gallate ZXHA-TC on Primary Osteoblasts
- Affiliations
-
- 1Department of Orthopedics, The First Hospital Affiliated to Henan University, Kaifeng, Henan, China.
- 2Guangxi Key Laboratory of Regenerative Medicine, Guangxi Medical University, Nanning, Guangxi, China. zhengli224@163.com, zhaojinmin@126.com
- 3Department of Orthopedic Trauma and Hand Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China.
- 4School of Chemistry and Chemical Engineering, Guangxi University, Nanning, Guangxi, China.
- 5Guangxi Key Laboratory of Traditional Chinese Medicine Quality Standards, Guangxi Institute of Traditional Medical and Pharmaceutical Sciences, Nanning, Guangxi, China.
- 6Department of Cell Biology & Genetics, School of Premedical Sciences, Guangxi Medical University, Nanning, Guangxi, China.
- 7The Medical and Scientific Research Center, Guangxi Medical University, Nanning, Guangxi, China.
- KMID: 2450351
- DOI: http://doi.org/10.3349/ymj.2015.56.3.760
Abstract
- PURPOSE
This study is intended to investigate the effects of plants or plant-derived antioxidants on prevention of osteoporosis through the maintenance of reactive oxygen species (ROS) at a favorable level.
MATERIALS AND METHODS
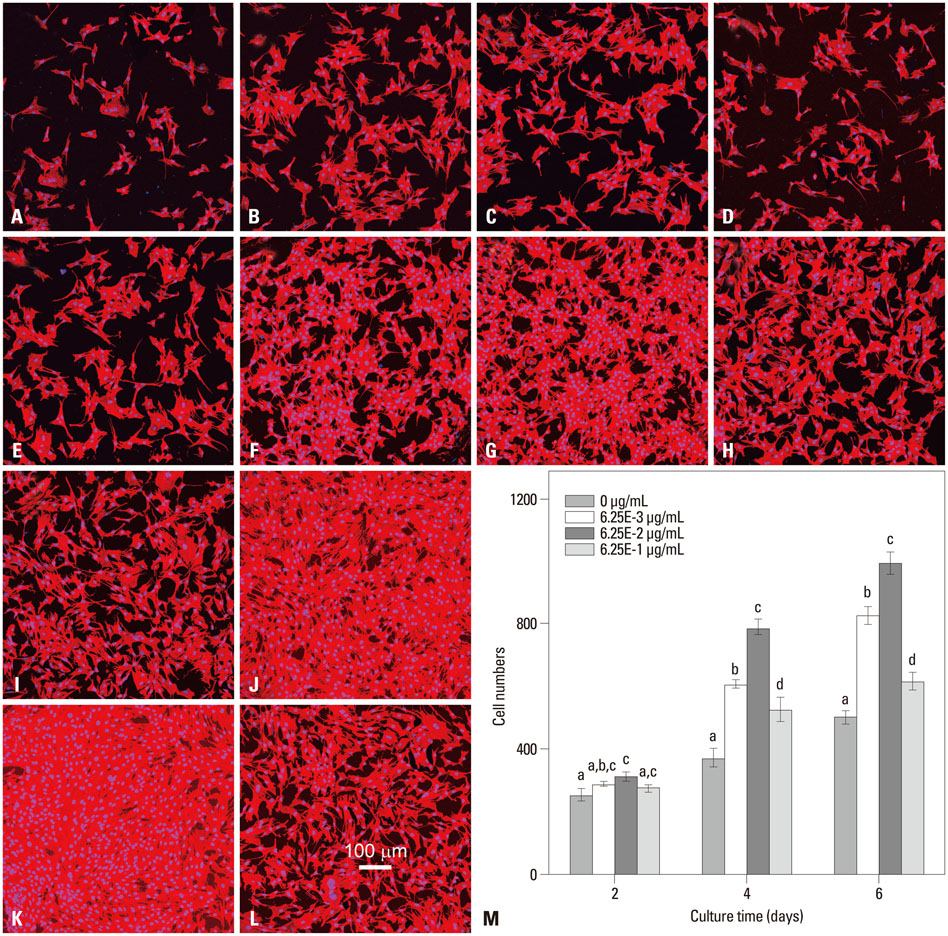
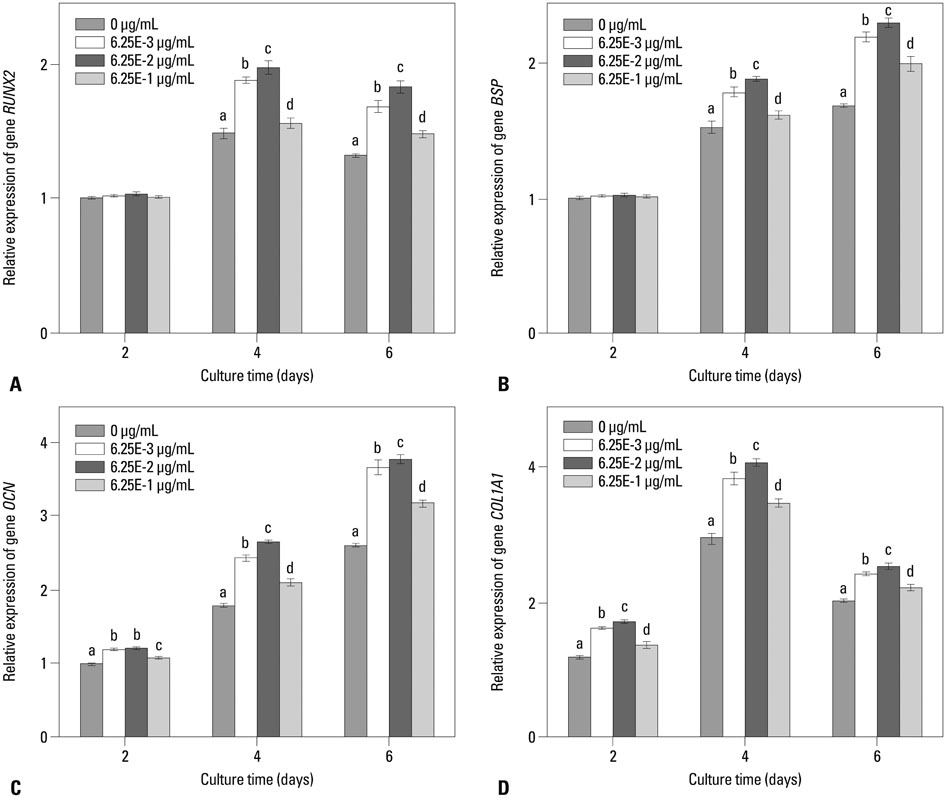
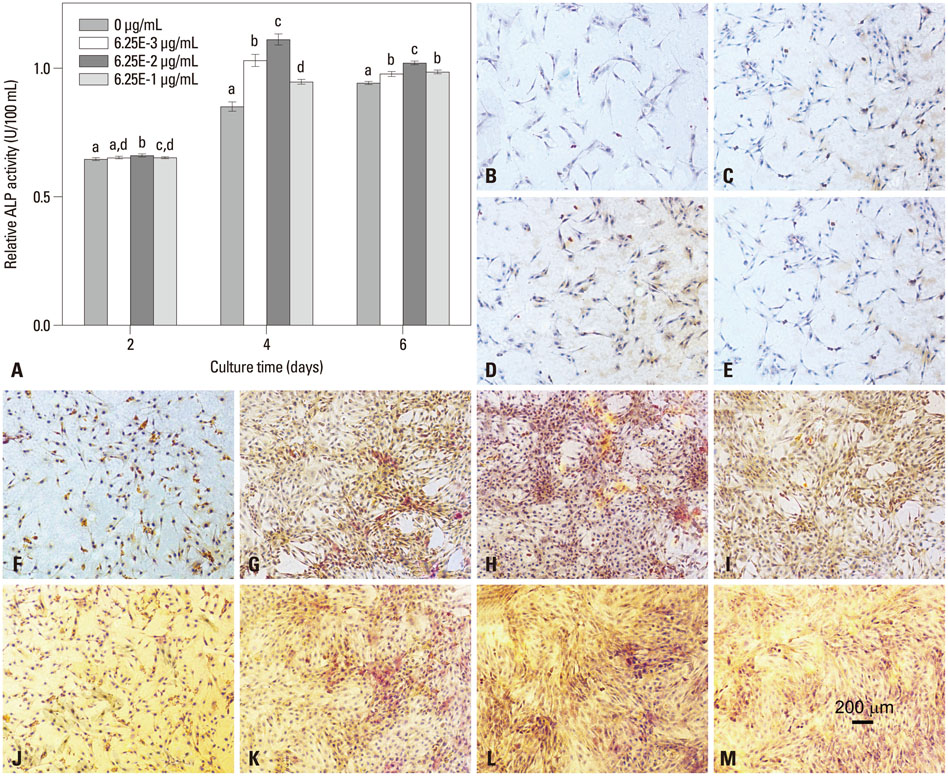
In this study, a novel antioxidant, namely 3,4,5-Trihydroxy-N-[4-(5-hydroxy-6-methoxy-pyrimidin-4-ylsulfamoyl)-phenyl]-benzamide (ZXHA-TC) was synthesized from gallic acid and sulfadimoxine. Its effect on osteoblast metabolism was investigated via the detection of cell proliferation, cell viability, production of ROS, and expression of osteogenic-specific genes including runt-related transcription factor 2 (RUNX2), bone sialoprotein (BSP), osteocalcin (OCN), alpha-1 type I collagen (COL1A1), and osteogenic-related proteins after treatment for 2, 4, and 6 days respectively.
RESULTS
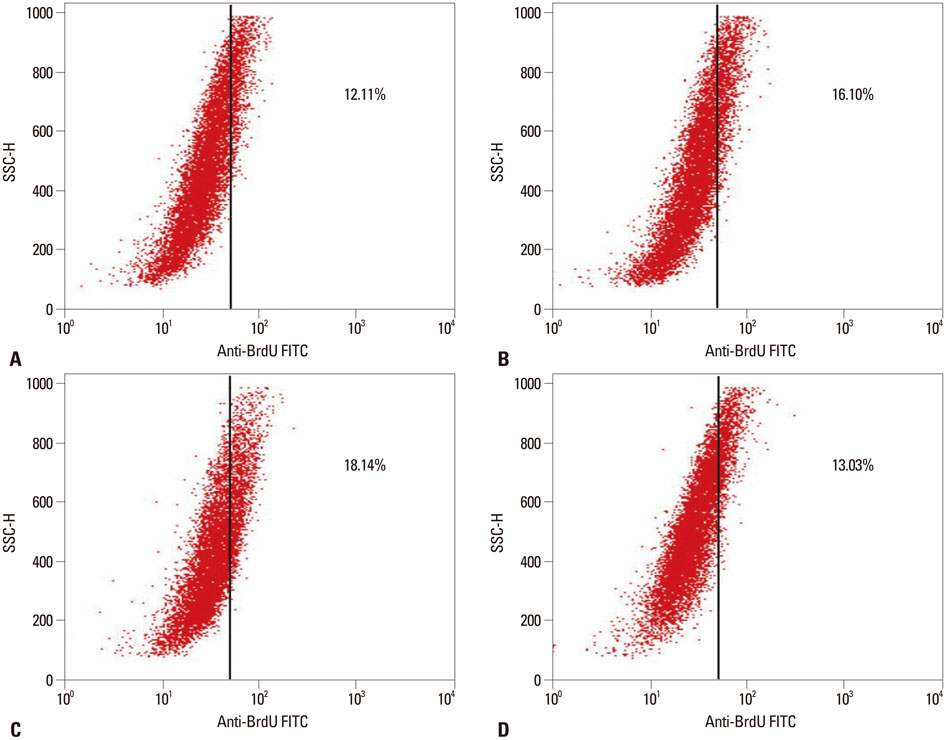
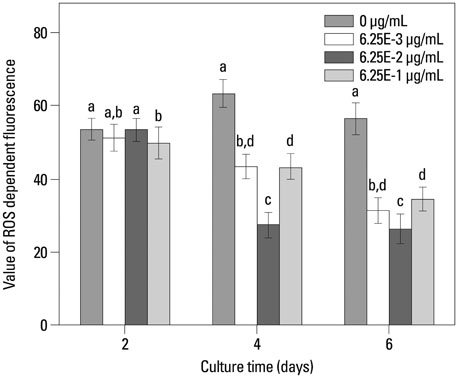
The results showed that ZXHA-TC has a stimulating effect on the proliferation and osteogenic differentiation of primary osteoblasts by promoting cell proliferation, cell viability, and the expression of genes BSP and OCN. Productions of bone matrix and mineralization were also increased by ZXHA-TC treatment as a result of up-regulation of COL1A1 and alkaline phosphatase (ALP) at the early stage and down-regulation of both genes subsequently. A range of 6.25x10(-3) microg/mL to 6.25x10(-1) microg/mL is the recommended dose for ZXHA-TC, within which 6.25x10(-2) microg/mL showed the best performance.
CONCLUSION
This study may hold promise for the development of a novel agent for the treatment of osteoporosis.
Keyword
MeSH Terms
-
Alkaline Phosphatase/metabolism
Bone Morphogenetic Proteins/pharmacology
Cell Differentiation/*drug effects
Cell Proliferation/*drug effects
Collagen Type I/genetics
Core Binding Factor Alpha 1 Subunit
Down-Regulation
Gallic Acid
Osteoblasts/*drug effects
Osteocalcin/metabolism
Osteogenesis/drug effects
Osteoporosis/*prevention & control
Reactive Oxygen Species
Up-Regulation
Alkaline Phosphatase
Bone Morphogenetic Proteins
Collagen Type I
Core Binding Factor Alpha 1 Subunit
Gallic Acid
Osteocalcin
Reactive Oxygen Species
Figure
Reference
-
1. Riggs BL, Melton LJ 3rd. Involutional osteoporosis. N Engl J Med. 1986; 314:1676–1686.
Article2. Simon LS. Osteoporosis. Rheum Dis Clin North Am. 2007; 33:149–176.
Article3. Andersen SJ. Osteoporosis in the older woman. Clin Obstet Gynecol. 2007; 50:752–766.
Article4. National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation;2010.5. National Osteoporosis Foundation. Bone Health Basics: Get the Facts. accessed on 2015 March 9. Available at: http://nof.org/learn/basics.6. National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation;2013.7. Johnell O. Advances in osteoporosis: better identification of risk factors can reduce morbidity and mortality. J Intern Med. 1996; 239:299–304.
Article8. Gold DT, Solimeo S. Osteoporosis and depression: a historical perspective. Curr Osteoporos Rep. 2006; 4:134–139.
Article9. Pereira JV, Modesto-Filho J, Agra MF, Barbosa-Filho JM. Plant and plant-derived compounds employed in prevention of the osteoporosis. Acta Farm Bonaerense. 2002; 21:223–234.10. Sharan K, Siddiqui JA, Swarnkar G, Maurya R, Chattopadhyay N. Role of phytochemicals in the prevention of menopausal bone loss: evidence from in vitro and in vivo, human interventional and pharma-cokinetic studies. Curr Med Chem. 2009; 16:1138–1157.
Article11. Arjmandi BH, Alekel L, Hollis BW, Amin D, Stacewicz-Sapuntzakis M, Guo P, et al. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr. 1996; 126:161–167.
Article12. Ren P, Ji H, Shao Q, Chen X, Han J, Sun Y. Protective effects of sodium daidzein sulfonate on trabecular bone in ovariectomized rats. Pharmacology. 2007; 79:129–136.
Article13. Kelly GE. Treatment or prevention of osteoporosis. U.S. Patent 6,340,703[P]. 2002. 01. 22.14. Yadav DK, Gautam AK, Kureel J, Srivastava K, Sahai M, Singh D, et al. Synthetic analogs of daidzein, having more potent osteoblast stimulating effect. Bioorg Med Chem Lett. 2011; 21:677–681.
Article15. Kuruto-Niwa R, Inoue S, Ogawa S, Muramatsu M, Nozawa R. Effects of tea catechins on the ERE-regulated estrogenic activity. J Agric Food Chem. 2000; 48:6355–6361.
Article16. Shen CL, Kwun IS, Wang S, Mo H, Chen L, Jenkins M, et al. Functions and mechanisms of green tea catechins in regulating bone remodeling. Curr Drug Targets. 2013; 14:1619–1630.
Article17. Lu Z, Nie G, Belton PS, Tang H, Zhao B. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int. 2006; 48:263–274.
Article18. Kroes BH, van den Berg AJ, Quarles van Ufford HC, van Dijk H, Labadie RP. Anti-inflammatory activity of gallic acid. Planta Med. 1992; 58:499–504.
Article19. Suntory Ltd. High level expression of proteins in yeast. Patent 6,183,985[P]. 1995. 03. 01.20. Ou TT, Lin MC, Wu CH, Lin WL, Wang CJ. Gallic acid attenuates oleic acid-induced proliferation of vascular smooth muscle cell through regulation of AMPK-eNOS-FAS signaling. Curr Med Chem. 2013; 20:3944–3953.
Article21. Yoon CH, Chung SJ, Lee SW, Park YB, Lee SK, Park MC. Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Joint Bone Spine. 2013; 80:274–279.
Article22. Chuang CY, Liu HC, Wu LC, Chen CY, Chang JT, Hsu SL. Gallic acid induces apoptosis of lung fibroblasts via a reactive oxygen species-dependent ataxia telangiectasia mutated-p53 activation pathway. J Agric Food Chem. 2010; 58:2943–2951.
Article23. Nuti E, Santamaria S, Casalini F, Yamamoto K, Marinelli L, La Pietra V, et al. Arylsulfonamide inhibitors of aggrecanases as potential therapeutic agents for osteoarthritis: synthesis and biological evaluation. Eur J Med Chem. 2013; 1:379–394.
Article24. Saxena HO, Faridi U, Srivastava S, Kumar JK, Darokar MP, Luqman S, et al. Gallic acid-based indanone derivatives as anticancer agents. Bioorg Med Chem Lett. 2008; 18:3914–3918.
Article25. Kang MS, Jang HS, Oh JS, Yang KH, Choi NK, Lim HS, et al. Effects of methyl gallate and gallic acid on the production of inflammatory mediators interleukin-6 and interleukin-8 by oral epithelial cells stimulated with Fusobacterium nucleatum. J Microbiol. 2009; 47:760–767.
Article26. Ho HH, Chang CS, Ho WC, Liao SY, Wu CH, Wang CJ. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-kappaB activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem Toxicol. 2010; 48:2508–2516.
Article27. Kuppan G, Balasubramanyam J, Monickaraj F, Srinivasan G, Mohan V, Balasubramanyam M. Transcriptional regulation of cytokines and oxidative stress by gallic acid in human THP-1 monocytes. Cytokine. 2010; 49:229–234.
Article28. Lo C, Lai TY, Yang JS, Yang JH, Ma YS, Weng SW, et al. Gallic acid inhibits the migration and invasion of A375.S2 human melanoma cells through the inhibition of matrix metalloproteinase-2 and Ras. Melanoma Res. 2011; 21:267–273.
Article29. Schroeder TM, Jensen ED, Westendorf JJ. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today. 2005; 75:213–225.
Article30. Cobb J, Dierich A, Huss-Garcia Y, Duboule D. A mouse model for human short-stature syndromes identifies Shox2 as an upstream regulator of Runx2 during long-bone development. Proc Natl Acad Sci U S A. 2006; 103:4511–4515.
Article31. Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, et al. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol. 2001; 155:157–166.
Article32. Na K, Kim SW, Sun BK, Woo DG, Yang HN, Chung HM, et al. Osteogenic differentiation of rabbit mesenchymal stem cells in thermo-reversible hydrogel constructs containing hydroxyapatite and bone morphogenic protein-2 (BMP-2). Biomaterials. 2007; 28:2631–2637.
Article33. Stein GS, Lian JB. Molecular mechanisms mediating proliferation/ differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993; 14:424–442.
Article34. D'Errico JA, MacNeil RL, Takata T, Berry J, Strayhorn C, Somerman MJ. Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone. 1997; 20:117–126.35. Hakki SS, Wang D, Franceschi RT, Somerman MJ. Bone sialoprotein gene transfer to periodontal ligament cells may not be sufficient to promote mineralization in vitro or in vivo. J Periodontol. 2006; 77:167–173.
Article36. Hakki SS, Bozkurt SB, Hakki EE, Belli S. Effects of mineral trioxide aggregate on cell survival, gene expression associated with mineralized tissues, and biomineralization of cementoblasts. J Endod. 2009; 35:513–519.
Article37. Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011; 54:1291–1297.
Article38. Tielens S, Wymeersch F, Declercq H, Cornelissen M. Effect of 17beta-estradiol on the in vitro differentiation of murine embryonic stem cells into the osteogenic lineage. In Vitro Cell Dev Biol Anim. 2008; 44:368–378.
Article39. zur Nieden NI, Kempka G, Ahr HJ. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation. 2003; 71:18–27.
Article40. Sugisawa A, Umegaki K. Physiological concentrations of (-)-epigallocatechin-3-O-gallate (EGCg) prevent chromosomal damage induced by reactive oxygen species in WIL2-NS cells. J Nutr. 2002; 132:1836–1839.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to “Stimulating Effect of a Novel Synthesized Sulfonamido-Based Gallate ZXHA-TC on Primary Osteoblasts†by Jin P, et al. (Yonsei Med J 2015;56(3):760-771.)
- Cytotoxic Effects of Gallic Acid and its Derivatives Against HIV-I-infected Microglia
- A Case of Allergic Contact Cheilitis from Propyl Gallate
- Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells
- Combined effect of recombinant human bone morphogenetic protein-2 and low level laser irradiation on bisphosphonate-treated osteoblasts