J Breast Cancer.
2019 Jun;22(2):237-247. 10.4048/jbc.2019.22.e29.
Cell Division Cycle Associated 8 Is a Key Regulator of Tamoxifen Resistance in Breast Cancer
- Affiliations
-
- 1Department of Breast Surgery, The People's Hospital of Cangzhou, Cangzhou, China. buyuhui2018@126.com
- KMID: 2450118
- DOI: http://doi.org/10.4048/jbc.2019.22.e29
Abstract
- PURPOSE
Breast cancer (BC) is one of the most common malignancies globally, and millions of women worldwide are diagnosed with BC every year. Up to 70% of BC patients are estrogen receptor (ER)-positive. Numerous studies have shown that tamoxifen has a significant therapeutic effect on both primary and metastatic ER-positive BC patients. Although tamoxifen is currently one of the most successful therapeutic agents for BC, a significant proportion of patients will eventually become resistant to tamoxifen, leading to tumor recurrence and metastasis. Knowledge about the development of tamoxifen resistance in BC patients is still limited.
METHODS
We applied a loss-and-gain method to study the biological functional role of cell division cycle associated 8 (CDCA8) in tamoxifen resistance in BC cells.
RESULTS
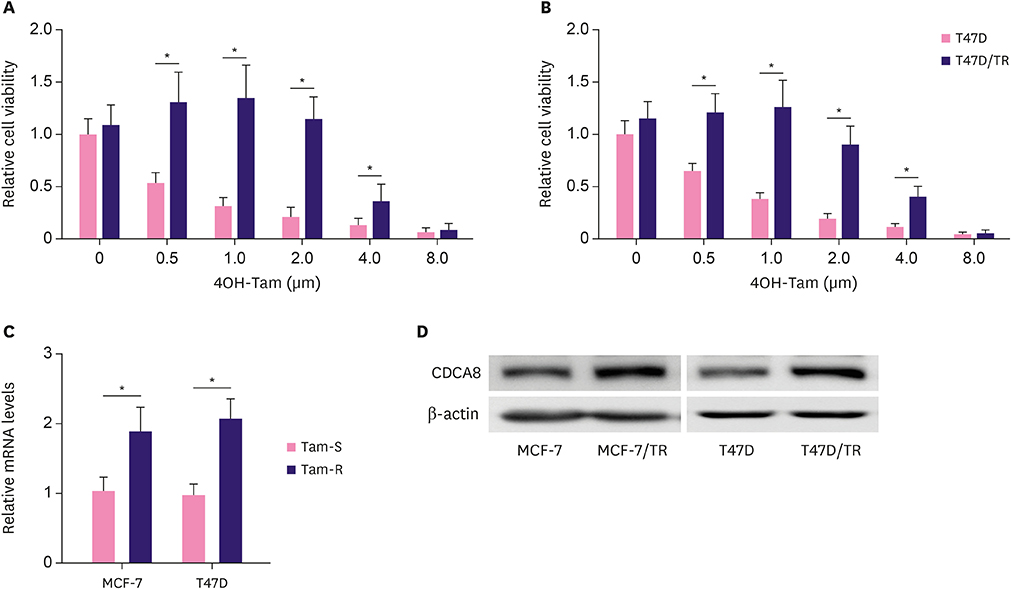
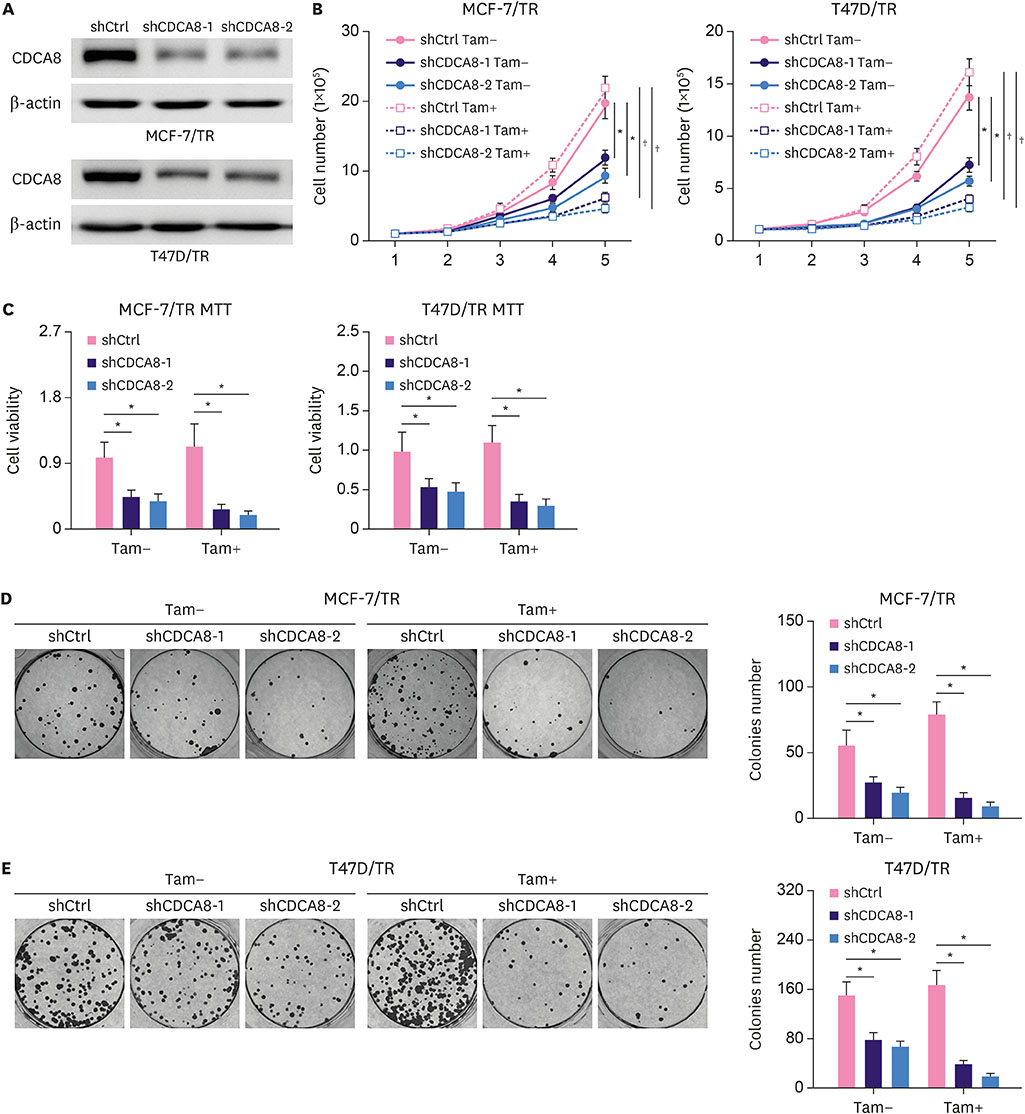
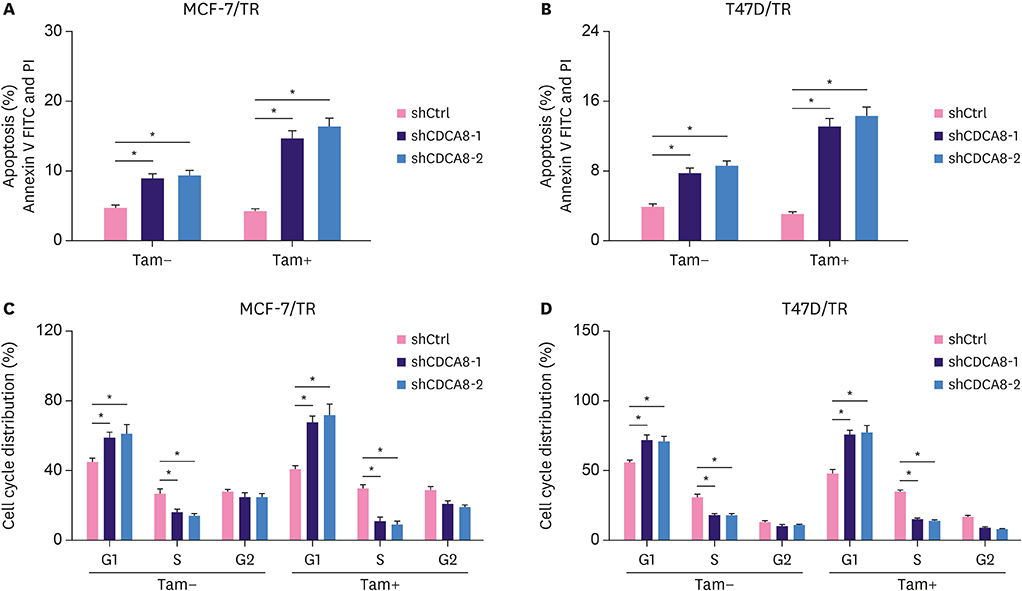
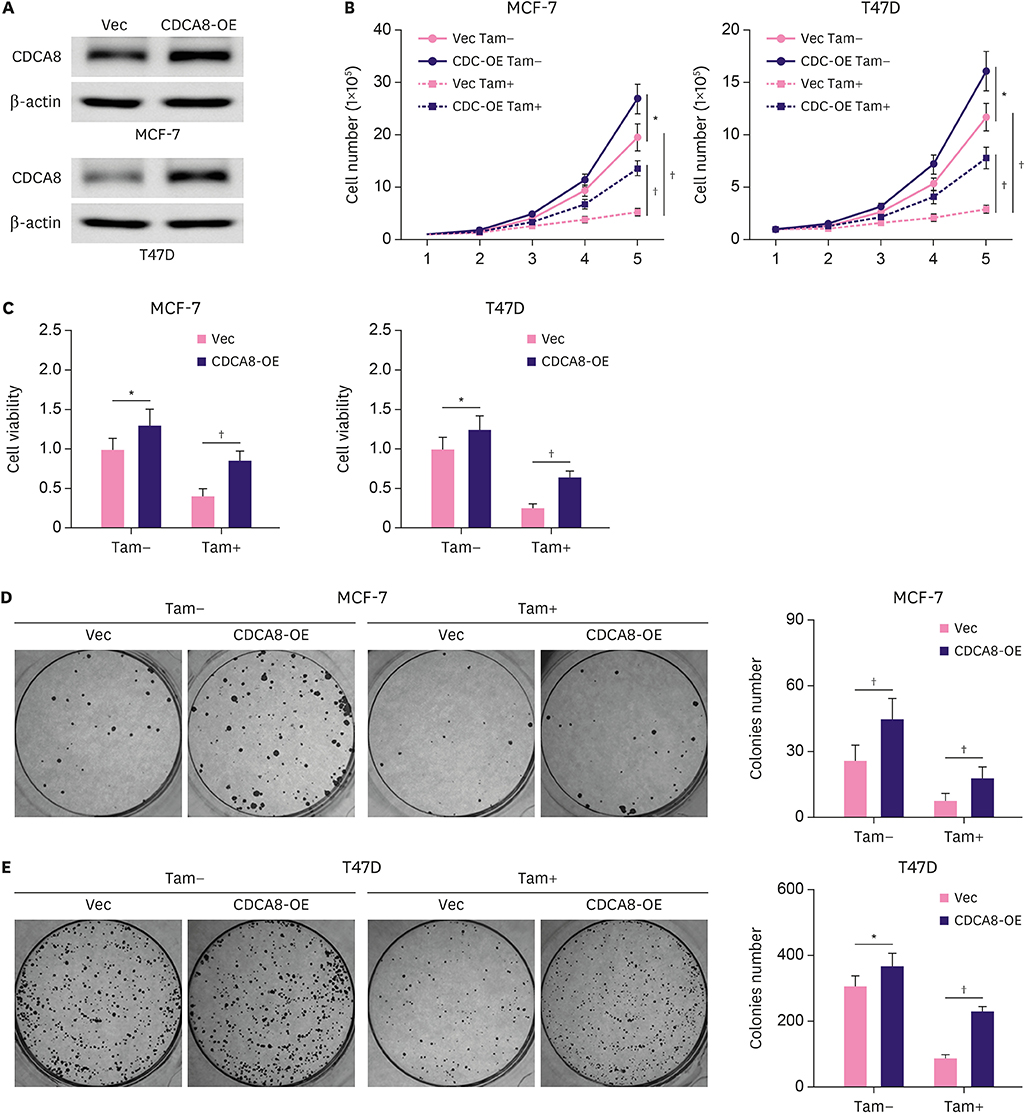
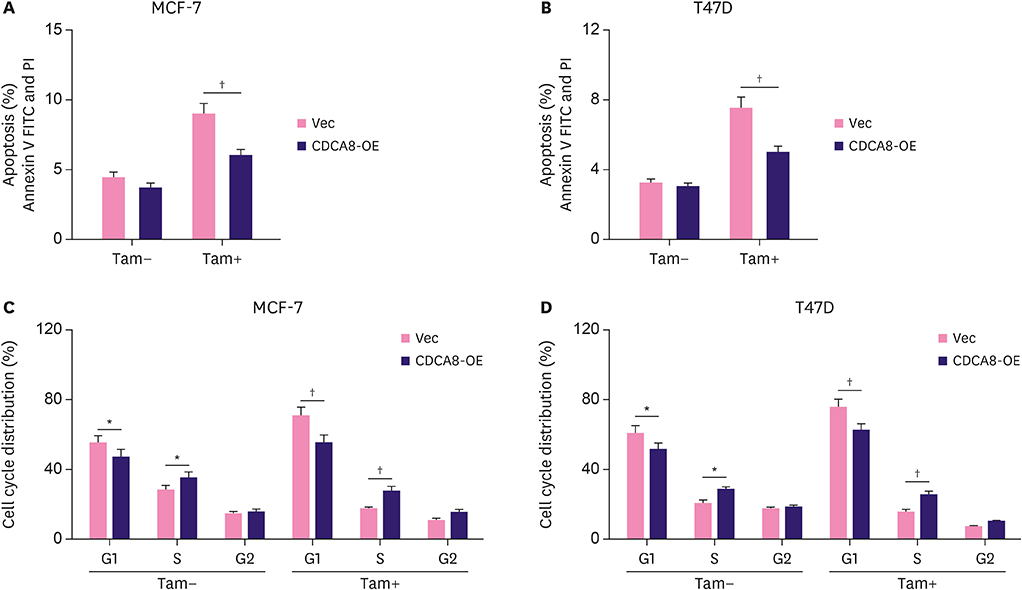
We found that CDCA8 was significantly elevated in tamoxifen-resistant BC cells. Knockdown of CDCA8 expression significantly inhibited the proliferation of tamoxifen-resistant BC cells and reduced their resistance to tamoxifen. In contrast, overexpression of CDCA8 promoted the growth of tamoxifen-sensitive BC cells and induced their resistance to tamoxifen.
CONCLUSION
In this study, we reported that CDCA8 is a key regulator of tamoxifen resistance in BC, suggesting that CDCA8 may serve as a potential therapeutic target for BC treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Seely JM, Alhassan T. Screening for breast cancer in 2018-what should we be doing today? Curr Oncol. 2018; 25:S115–S124.
Article2. Wu TY, Liu YL, Chung S. Improving breast cancer outcomes among women in China: practices, knowledge, and attitudes related to breast cancer screening. Int J Breast Cancer. 2012; 2012:921607.
Article3. Chang M. Tamoxifen resistance in breast cancer. Biomol Ther (Seoul). 2012; 20:256–267.
Article4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674.
Article5. Labi V, Erlacher M. How cell death shapes cancer. Cell Death Dis. 2015; 6:e1675.
Article6. Giam M, Rancati G. Aneuploidy and chromosomal instability in cancer: a jackpot to chaos. Cell Div. 2015; 10:3.
Article7. Ly P, Cleveland DW. Interrogating cell division errors using random and chromosome-specific missegregation approaches. Cell Cycle. 2017; 16:1252–1258.
Article8. Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007; 8:798–812.
Article9. Haase J, Bonner MK, Halas H, Kelly AE. Distinct roles of the chromosomal passenger complex in the detection of and response to errors in kinetochore-microtubule attachment. Dev Cell. 2017; 42:640–654.e5.
Article10. Carmena M, Wheelock M, Funabiki H, Earnshaw WC. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol. 2012; 13:789–803.
Article11. Phan NN, Wang CY, Li KL, Chen CF, Chiao CC, Yu HG, et al. Distinct expression of CDCA3, CDCA5, and CDCA8 leads to shorter relapse free survival in breast cancer patient. Oncotarget. 2018; 9:6977–6992.
Article12. Bi Y, Chen S, Jiang J, Yao J, Wang G, Zhou Q, et al. CDCA8 expression and its clinical relevance in patients with bladder cancer. Medicine (Baltimore). 2018; 97:e11899.
Article13. Chang JL, Chen TH, Wang CF, Chiang YH, Huang YL, Wong FH, et al. Borealin/Dasra B is a cell cycle-regulated chromosomal passenger protein and its nuclear accumulation is linked to poor prognosis for human gastric cancer. Exp Cell Res. 2006; 312:962–973.
Article14. Hayama S, Daigo Y, Yamabuki T, Hirata D, Kato T, Miyamoto M, et al. Phosphorylation and activation of cell division cycle associated 8 by aurora kinase B plays a significant role in human lung carcinogenesis. Cancer Res. 2007; 67:4113–4122.
Article15. Ju W, Yoo BC, Kim IJ, Kim JW, Kim SC, Lee HP. Identification of genes with differential expression in chemoresistant epithelial ovarian cancer using high-density oligonucleotide microarrays. Oncol Res. 2009; 18:47–56.
Article16. Narayan G, Bourdon V, Chaganti S, Arias-Pulido H, Nandula SV, Rao PH, et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: identification of candidate amplified and overexpressed genes. Genes Chromosomes Cancer. 2007; 46:373–384.
Article17. Ci C, Tang B, Lyu D, Liu W, Qiang D, Ji X, et al. Overexpression of CDCA8 promotes the malignant progression of cutaneous melanoma and leads to poor prognosis. Int J Mol Med. 2019; 43:404–412.
Article18. Zhu Y, Liu Y, Zhang C, Chu J, Wu Y, Li Y, et al. Tamoxifen-resistant breast cancer cells are resistant to DNA-damaging chemotherapy because of upregulated BARD1 and BRCA1. Nat Commun. 2018; 9:1595.
Article19. Lü M, Ding K, Zhang G, Yin M, Yao G, Tian H, et al. MicroRNA-320a sensitizes tamoxifen-resistant breast cancer cells to tamoxifen by targeting ARPP-19 and ERRγ. Sci Rep. 2015; 5:8735.
Article20. Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006; 102:89–96.
Article21. Russo J, Ao X, Grill C, Russo IH. Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat. 1999; 53:217–227.
Article22. Chang XZ, Li DQ, Hou YF, Wu J, Lu JS, Di GH, et al. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007; 9:R76.
Article23. Viedma-Rodríguez R, Baiza-Gutman L, Salamanca-Gómez F, Diaz-Zaragoza M, Martínez-Hernández G, Ruiz Esparza-Garrido R, et al. Mechanisms associated with resistance to tamoxifen in estrogen receptor-positive breast cancer (review). Oncol Rep. 2014; 32:3–15.
Article24. Bostner J, Karlsson E, Pandiyan MJ, Westman H, Skoog L, Fornander T, et al. Activation of Akt, mTOR, and the estrogen receptor as a signature to predict tamoxifen treatment benefit. Breast Cancer Res Treat. 2013; 137:397–406.
Article25. Hultsch S, Kankainen M, Paavolainen L, Kovanen RM, Ikonen E, Kangaspeska S, et al. Association of tamoxifen resistance and lipid reprogramming in breast cancer. BMC Cancer. 2018; 18:850.
Article26. Ojo D, Wei F, Liu Y, Wang E, Zhang H, Lin X, et al. Factors promoting tamoxifen resistance in breast cancer via stimulating breast cancer stem cell expansion. Curr Med Chem. 2015; 22:2360–2374.
Article27. Kilker RL, Planas-Silva MD. Cyclin D1 is necessary for tamoxifen-induced cell cycle progression in human breast cancer cells. Cancer Res. 2006; 66:11478–11484.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tamoxifen Resistance and Crosstalk of Signal Transduction in Breast Cancer
- Mammographic Changes in Breast Cancer Patients Treated with Tamoxifen
- Inhibition of EGFR and HER2 in Tamoxifen-resistant T47D:A18/4-OHT Breast Cancer Cells
- Cyclin D1 Expression and Patient Outcome After Tamoxifen Therapy in Estrogen Receptor Positive Breast Cancer
- Two Cases of Endometrial Carcinoma in Tamoxifen-treated Breast Cancer Patients in Korea