J Breast Cancer.
2019 Jun;22(2):185-195. 10.4048/jbc.2019.22.e22.
Doxorubicin Promotes Migration and Invasion of Breast Cancer Cells through the Upregulation of the RhoA/MLC Pathway
- Affiliations
-
- 1Department of Surgery, MacKay Memorial Hospital and Mackay Medical College, Taipei, Taiwan. changyc@mmh.org.tw
- 2Department of Medical Research, MacKay Memorial Hospital, Taipei, Taiwan.
- 3Department of Bioscience Technology, Chung Yuan Christian University, Taoyuan City, Taiwan.
- KMID: 2450114
- DOI: http://doi.org/10.4048/jbc.2019.22.e22
Abstract
- PURPOSE
Cancer cells develop acquired resistance induced by chemotherapeutic drugs. In this study, we investigated the effects of brief treatment with cytotoxic drugs on the phenotype of breast cancer cells.
METHODS
Breast cancer cells MCF7 and BT-474 were briefly treated with paclitaxel or doxorubicin. Clonogenic, migration, and invasion assays were performed on the treated cells. Western blot analysis and RhoA activity assay were also performed.
RESULTS
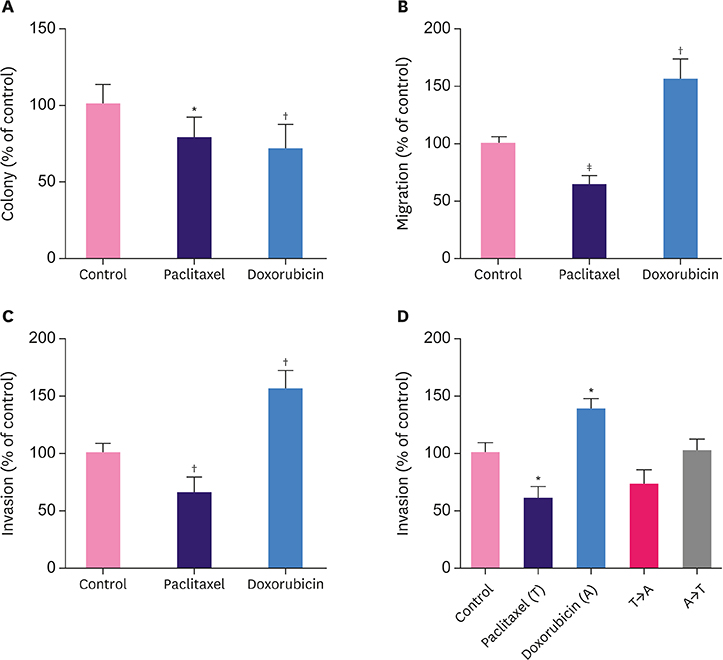
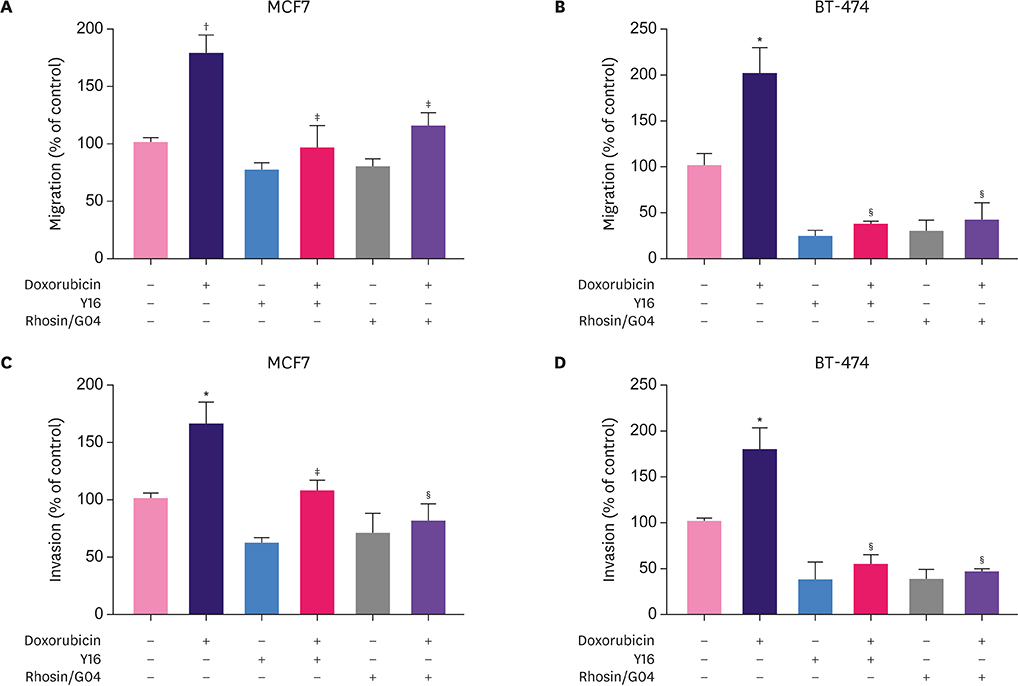
Breast cancer cells when briefly treated with paclitaxel or doxorubicin showed reduced clonogenic ability. Doxorubicin, but not paclitaxel, augmented cell migration and invasion. The invasion-promoting effects of doxorubicin were lost when the two drugs were sequentially used in combination. Myosin light chain (MLC) 2 phosphorylation and RhoA activity were upregulated by doxorubicin and downregulated by paclitaxel. Pretreatment with RhoA inhibitors abolished the migration- and invasion-promoting effects of doxorubicin.
CONCLUSION
Doxorubicin activates the RhoA/MLC pathway and enhances breast cancer cell migration and invasion. Therefore, this pathway might be explored as a therapeutic target to suppress anthracycline-enhanced tumor progression.
Keyword
MeSH Terms
Figure
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.
Article2. Hart CD, Sanna G, Siclari O, Biganzoli L, Di Leo A. Defining optimal duration and predicting benefit from chemotherapy in patients with luminal-like subtypes. Breast. 2015; 24:Suppl 2. S136–S142.
Article3. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012; 379:432–444.
Article4. Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst. 2014; 106:dju289.
Article5. Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, Jukkola-Vuorinen A, Tanner M, Asola R, et al. Adjuvant capecitabine in combination with docetaxel and cyclophosphamide plus epirubicin for breast cancer: an open-label, randomised controlled trial. Lancet Oncol. 2009; 10:1145–1151.
Article6. Wurz GT, DeGregorio MW. Activating adaptive cellular mechanisms of resistance following sublethal cytotoxic chemotherapy: implications for diagnostic microdosing. Int J Cancer. 2015; 136:1485–1493.
Article7. Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008; 100:672–679.
Article8. Hanauske AR, Hanauske U, Von Hoff DD. The human tumor cloning assay in cancer research and therapy: a review with clinical correlations. Curr Probl Cancer. 1985; 9:1–50.
Article9. Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC, Cheng SP. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS One. 2014; 9:e89563.
Article10. Chang YC, Chen CK, Chen MJ, Lin JC, Lin CH, Huang WC, et al. Expression of 3β-hydroxysteroid dehydrogenase type 1 in breast cancer is associated with poor prognosis independent of estrogen receptor status. Ann Surg Oncol. 2017; 24:4033–4041.
Article11. Chang YC, Liu CL, Chen MJ, Hsu YW, Chen SN, Lin CH, et al. Local anesthetics induce apoptosis in human breast tumor cells. Anesth Analg. 2014; 118:116–124.
Article12. Shang X, Marchioni F, Sipes N, Evelyn CR, Jerabek-Willemsen M, Duhr S, et al. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem Biol. 2012; 19:699–710.
Article13. Menna P, Salvatorelli E. Primary prevention strategies for anthracycline cardiotoxicity: a brief overview. Chemotherapy. 2017; 62:159–168.
Article14. Dalmases A, González I, Menendez S, Arpí O, Corominas JM, Servitja S, et al. Deficiency in p53 is required for doxorubicin induced transcriptional activation of NF-кB target genes in human breast cancer. Oncotarget. 2014; 5:196–210.
Article15. Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q, Tang F, et al. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res. 2009; 15:2657–2665.
Article16. Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009; 10:778–790.
Article17. Ebata T, Mitsui Y, Sugimoto W, Maeda M, Araki K, Machiyama H, et al. Substrate stiffness influences doxorubicin-induced p53 activation via ROCK2 expression. BioMed Res Int. 2017; 2017:5158961.
Article18. Zandvakili I, Lin Y, Morris JC, Zheng Y. Rho GTPases: anti- or pro-neoplastic targets? Oncogene. 2017; 36:3213–3222.
Article19. Fujii T, Le Du F, Xiao L, Kogawa T, Barcenas CH, Alvarez RH, et al. Effectiveness of an adjuvant chemotherapy regimen for early-stage breast cancer: a systematic review and network meta-analysis. JAMA Oncol. 2015; 1:1311–1318.
Article20. Simon R, Norton L. The Norton-Simon hypothesis: designing more effective and less toxic chemotherapeutic regimens. Nat Clin Pract Oncol. 2006; 3:406–407.
Article21. Wildiers H, Dirix L, Neven P, Prové A, Clement P, Squifflet P, et al. Delivery of adjuvant sequential dose-dense FEC-Doc to patients with breast cancer is feasible, but dose reductions and toxicity are dependent on treatment sequence. Breast Cancer Res Treat. 2009; 114:103–112.
Article22. Piedbois P, Serin D, Priou F, Laplaige P, Greget S, Angellier E, et al. Dose-dense adjuvant chemotherapy in node-positive breast cancer: docetaxel followed by epirubicin/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Ann Oncol. 2007; 18:52–57.
Article23. Cardoso F, Ferreira Filho AF, Crown J, Dolci S, Paesmans M, Riva A, et al. Doxorubicin followed by docetaxel versus docetaxel followed by doxorubicin in the adjuvant treatment of node positive breast cancer: results of a feasibility study. Anticancer Res. 2001; 21:789–795.24. Earl HM, Vallier AL, Hiller L, Fenwick N, Young J, Iddawela M, et al. Effects of the addition of gemcitabine, and paclitaxel-first sequencing, in neoadjuvant sequential epirubicin, cyclophosphamide, and paclitaxel for women with high-risk early breast cancer (Neo-tAnGo): an open-label, 2×2 factorial randomised phase 3 trial. Lancet Oncol. 2014; 15:201–212.
Article25. Guo B, Villeneuve DJ, Hembruff SL, Kirwan AF, Blais DE, Bonin M, et al. Cross-resistance studies of isogenic drug-resistant breast tumor cell lines support recent clinical evidence suggesting that sensitivity to paclitaxel may be strongly compromised by prior doxorubicin exposure. Breast Cancer Res Treat. 2004; 85:31–51.
Article26. Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007; 1773:642–652.
Article27. Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007; 117:1305–1313.
Article28. Yeh PY, Lu YS, Ou DL, Cheng AL. IκB kinases increase Myc protein stability and enhance progression of breast cancer cells. Mol Cancer. 2011; 10:53.
Article29. Dominguez R, Holmes KC. Actin structure and function. Annu Rev Biophys. 2011; 40:169–186.
Article30. Katoh K, Kano Y, Noda Y. Rho-associated kinase-dependent contraction of stress fibres and the organization of focal adhesions. J R Soc Interface. 2011; 8:305–311.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resveratrol suppresses breast cancer cell invasion by inactivating a RhoA/YAP signaling axis
- The Cancer/Testis Antigen CT45A1 Promotes Transcription of Oncogenic Sulfatase-2 Gene in Breast Cancer Cells and Is Sensible Targets for Cancer Therapy
- LETM1 Promotes Gastric Cancer Cell Proliferation, Migration, and Invasion via the PI3K/Akt Signaling Pathway
- Prolactin Inhibits BCL6 Expression in Breast Cancer Cells through a MicroRNA-339-5p-Dependent Pathway
- Enhancing the Effects of Low Dose Doxorubicin Treatment by the Radiation in T47D and SKBR3 Breast Cancer Cells