Int J Thyroidol.
2018 Nov;11(2):71-74. 10.11106/ijt.2018.11.2.71.
Management of Bleeding Induced by Tyrosine Kinase Inhibitor in Radioiodine Refractory Thyroid Cancer
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. shindongyi@yuhs.ac
- KMID: 2448973
- DOI: http://doi.org/10.11106/ijt.2018.11.2.71
Abstract
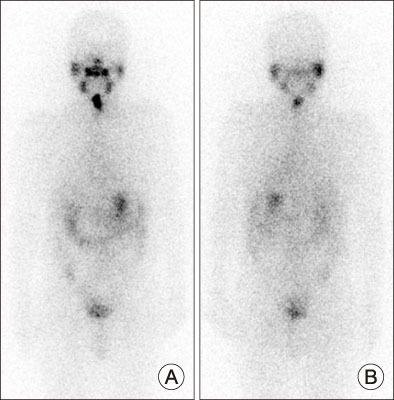
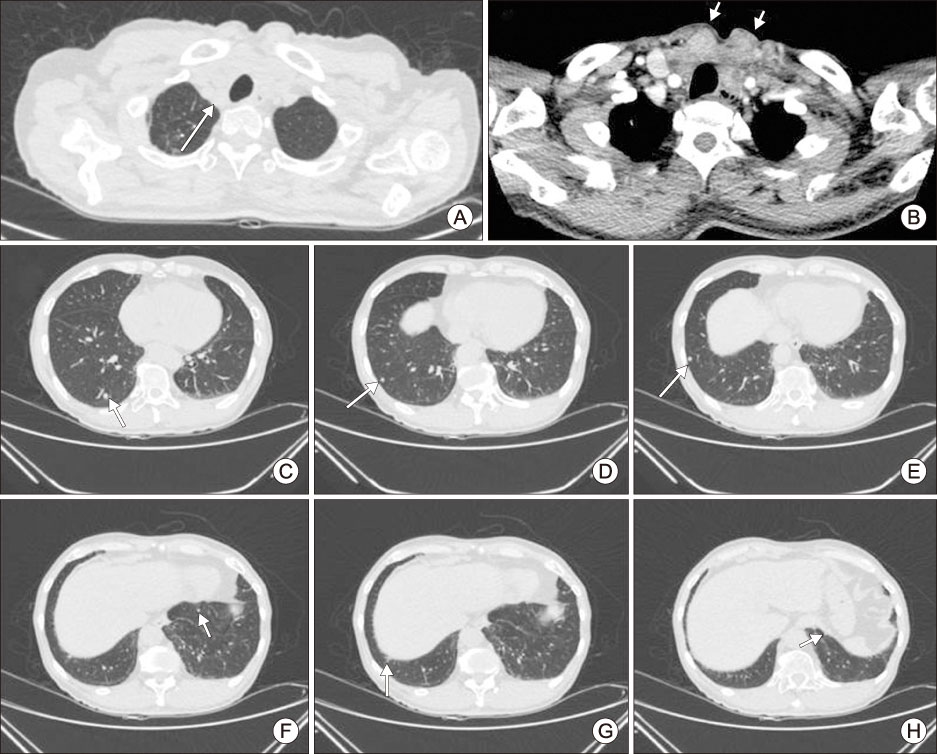
- Adverse events such as hemoptysis and gastrointestinal hemorrhage during tyrosine kinase inhibitor treatment are relatively rare, but the severity of the bleeding can be higher than other common adverse events. It is necessary to educate patients about its possibility so that they can be found early. In this case report of radioiodine refractory thyroid cancer patient, hemoptysis and gastrointestinal bleeding has occurred following lenvatinib administration. Drug interruption and dose modification and dose interruption were required in addition to management for bleeding itself. It is necessary to confirm the high risk of bleeding before the administration of tyrosine kinase inhibitors, and to appropriately control the follow-up interval and drug dosage accordingly.
MeSH Terms
Figure
Reference
-
1. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014; 384(9940):319–328.
Article2. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodinerefractory thyroid cancer. N Engl J Med. 2015; 372(7):621–630.
Article3. Launay-Vacher V, Deray G. Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anticancer Drugs. 2009; 20(1):81–82.
Article4. Blevins DP, Dadu R, Hu M, Baik C, Balachandran D, Ross W, et al. Aerodigestive fistula formation as a rare side effect of antiangiogenic tyrosine kinase inhibitor therapy for thyroid cancer. Thyroid. 2014; 24(5):918–922.
Article5. Lamartina L, Ippolito S, Danis M, Bidault F, Borget I, Berdelou A, et al. Antiangiogenic tyrosine kinase inhibitors: occurrence and risk factors of hemoptysis in refractory thyroid cancer. J Clin Endocrinol Metab. 2016; 101(7):2733–2741.
Article6. Barber NA, Afzal W, Akhtari M. Hematologic toxicities of small molecule tyrosine kinase inhibitors. Target Oncol. 2011; 6(4):203–215.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Severe Fatigue Induced by Tyrosine Kinase Inhibitor in Radioiodine Refractory Thyroid Cancer
- Skin-Related Toxicity of Tyrosine Kinase Inhibitor in Thyroid Cancer
- Current Status and Future Perspective of the Treatment for Radioiodine Refractory Differentiated Thyroid Cancer
- Molecular targeted therapy of thyroid cancer
- Adverse Events of Tyrosine Kinase Inhibitors in Patients with Advanced Thyroid Cancer