Int J Thyroidol.
2018 Nov;11(2):75-77. 10.11106/ijt.2018.11.2.75.
Management of Severe Fatigue Induced by Tyrosine Kinase Inhibitor in Radioiodine Refractory Thyroid Cancer
- Affiliations
-
- 1Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Daegu, Korea. abc2000@knu.ac.kr
- KMID: 2448974
- DOI: http://doi.org/10.11106/ijt.2018.11.2.75
Abstract
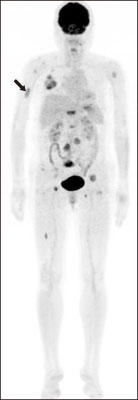
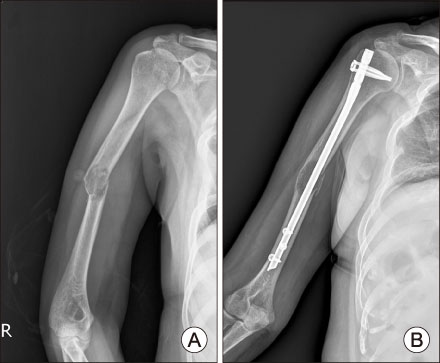
- Tyrosine kinase inhibitor is known to prolong progression free survival in radioiodine refractory thyroid cancer patients. Fatigue/asthenia/malaise is one of most common adverse events by the tyrosine kinase inhibitor treatment, and management of the adverse event is important to keep the drug medication longer which is essential for the survival benefit. In the case report, a radioiodine refractory thyroid cancer patient receiving tyrosine kinase inhibitor experienced severe fatigue, and a pathologic fracture of right humerus occurred by slipping down which was tightly linked with the adverse event of the drug. The pathologic fracture was surgically well managed and the adverse event was well controlled by supportive managements combined with dose reduction of the tyrosine kinase inhibitor. The drug administration to the patient was kept more than 1 year without progression of the disease.
MeSH Terms
Figure
Reference
-
1. Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, et al. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016; 17(9):1272–1282.
Article2. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015; 372(7):621–630.
Article3. Takahashi S, Kiyota N, Tahara M. Optimal use of lenvatinib in the treatment of advanced thyroid cancer. Cancers Head Neck. 2017; 2:7.
Article4. Brose MS, Frenette CT, Keefe SM, Stein SM. Management of sorafenib-related adverse events: a clinician's perspective. Semin Oncol. 2014; 41:Suppl 2. S1–S16.
Article5. Worden F, Fassnacht M, Shi Y, Hadjieva T, Bonichon F, Gao M, et al. Safety and tolerability of sorafenib in patients with radioiodine-refractory thyroid cancer. Endocr Relat Cancer. 2015; 22(6):877–887.
Article6. Chrisoulidou A, Mandanas S, Margaritidou E, Mathiopoulou L, Boudina M, Georgopoulos K, et al. Treatment compliance and severe adverse events limit the use of tyrosine kinase inhibitors in refractory thyroid cancer. Onco Targets Ther. 2015; 8:2435–2442.7. Resteghini C, Cavalieri S, Galbiati D, Granata R, Alfieri S, Bergamini C, et al. Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best Pract Res Clin Endocrinol Metab. 2017; 31(3):349–361.
Article8. Haddad RI, Schlumberger M, Wirth LJ, Sherman EJ, Shah MH, Robinson B, et al. Incidence and timing of common adverse events in Lenvatinib-treated patients from the SELECT trial and their association with survival outcomes. Endocrine. 2017; 56(1):121–128.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Bleeding Induced by Tyrosine Kinase Inhibitor in Radioiodine Refractory Thyroid Cancer
- Skin-Related Toxicity of Tyrosine Kinase Inhibitor in Thyroid Cancer
- Current Status and Future Perspective of the Treatment for Radioiodine Refractory Differentiated Thyroid Cancer
- Molecular targeted therapy of thyroid cancer
- A Case Report of Severe Hypocalcemia and Hypothyroidism after Tyrosine Kinase Inhibitor Treatment