Allergy Asthma Immunol Res.
2019 Jul;11(4):548-559. 10.4168/aair.2019.11.4.548.
Combination Effect of Titrated Extract of Centella asiatica and Astaxanthin in a Mouse Model of Phthalic Anhydride-Induced Atopic Dermatitis
- Affiliations
-
- 1College of Pharmacy and Medical Research Center, Chungbuk National University, Cheongju, Korea. jinthong@chungbuk.ac.kr
- KMID: 2448757
- DOI: http://doi.org/10.4168/aair.2019.11.4.548
Abstract
- PURPOSE
In our previous study, we demonstrated that both titrated extract of Centella asiatica (TECA) and astaxanthin (AST) have anti-inflammatory effects in a 5% phthalic anhydride (PA) mouse model of atopic dermatitis (AD). The increasing prevalence of AD demands new therapeutic approaches for treating the disease. We investigated the therapeutic efficacy of the ointment form of TECA, AST and a TECA + AST combination in a mouse model of AD to see whether a combination of the reduced doses of 2 compounds could have a synergistic effect.
METHODS
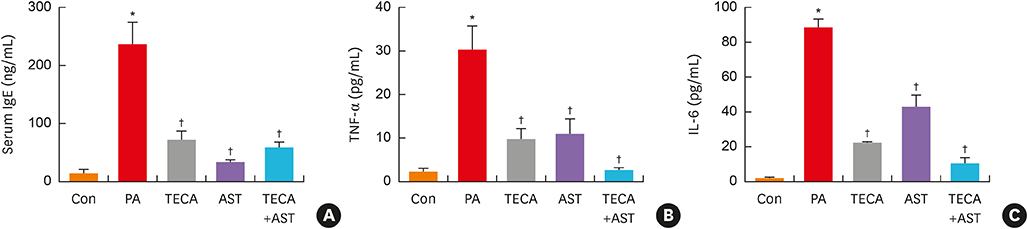
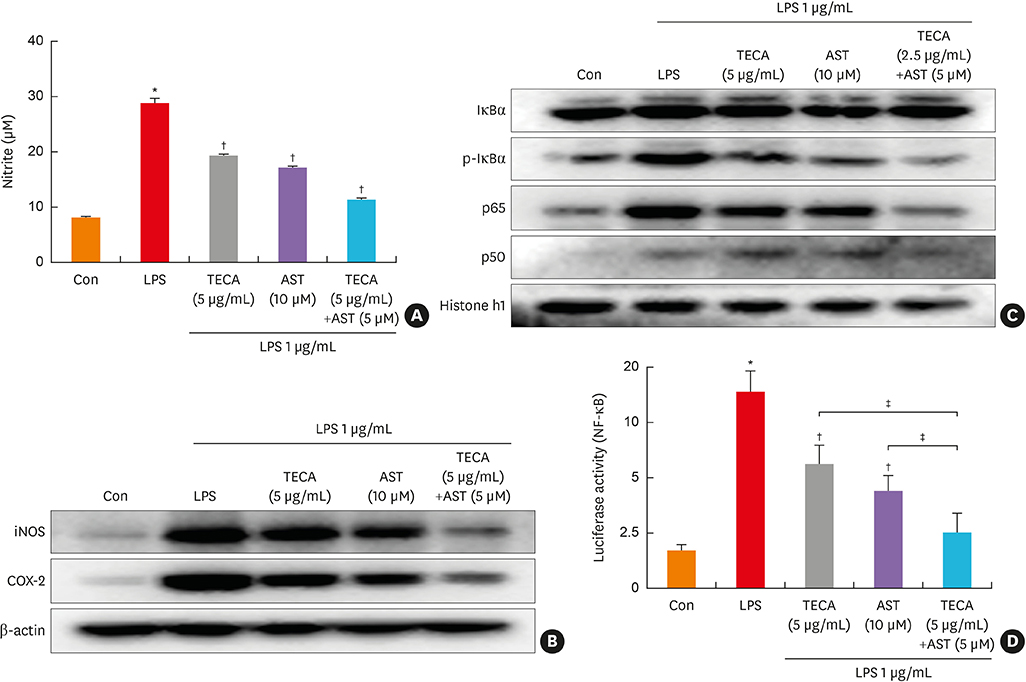
An AD-like lesion was induced by the topical application of 5% PA to the dorsal ear and back skin of an Hos:HR-1 mouse. After AD induction, TECA (0.5%), AST (0.5%) and the TECA (0.25%) + AST (0.25%) combination ointment (20 μg/cm2) were spread on the dorsum of the ear or back skin 3 times a week for 4 weeks. We evaluated dermatitis severity, histopathological changes and changes in protein expression by Western blotting for inducible nitric oxide synthase (iNOS), cyclocxygenase (COX)-2, and nuclear factor (NF)-κB activity. We also measured the concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and immunoglobulin E (IgE) in the blood of AD mice by enzyme-linked immunosorbent assay (ELISA).
RESULTS
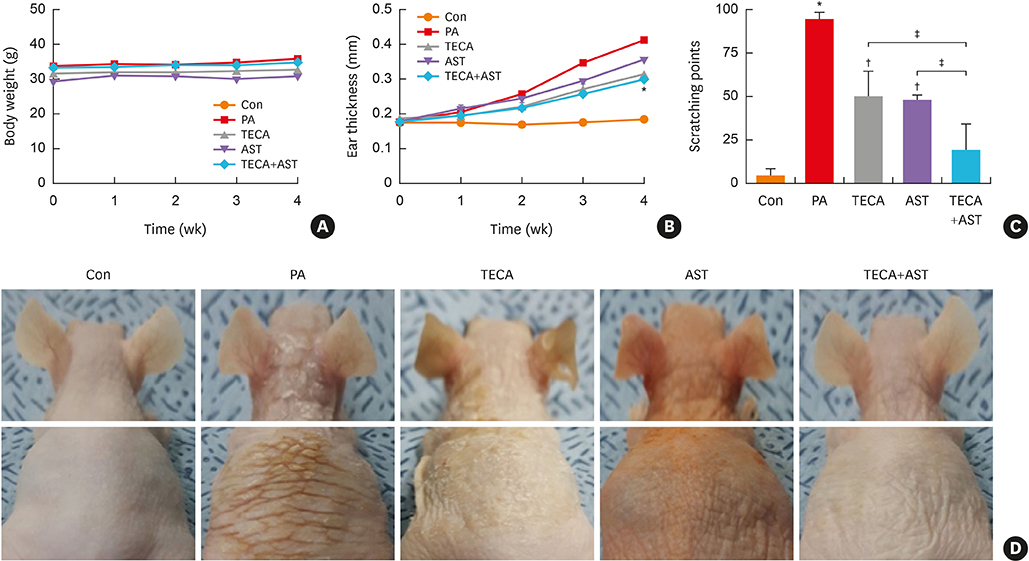
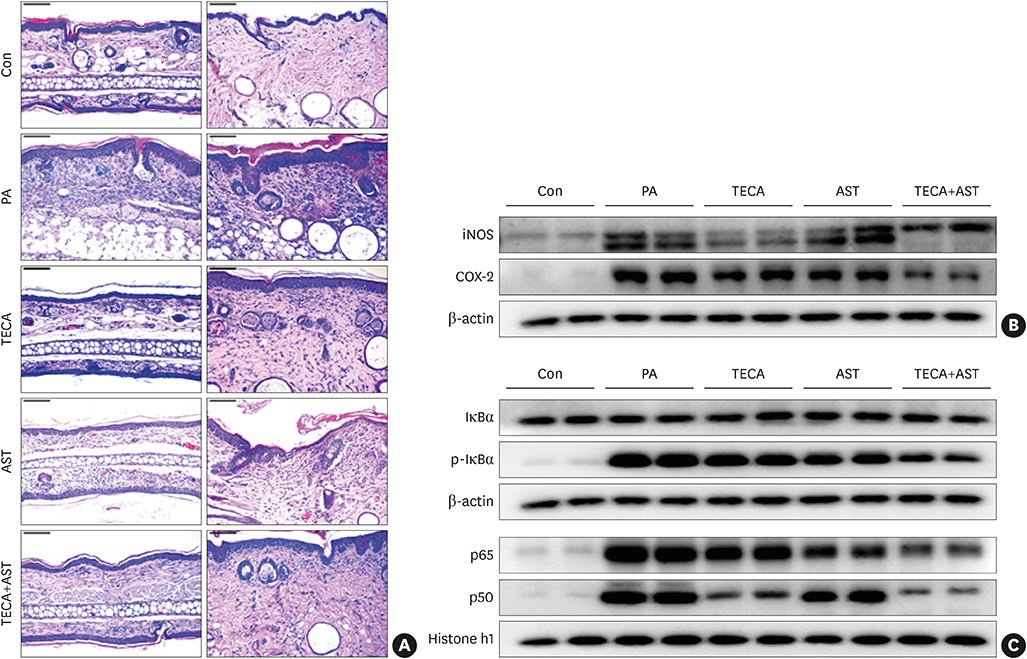
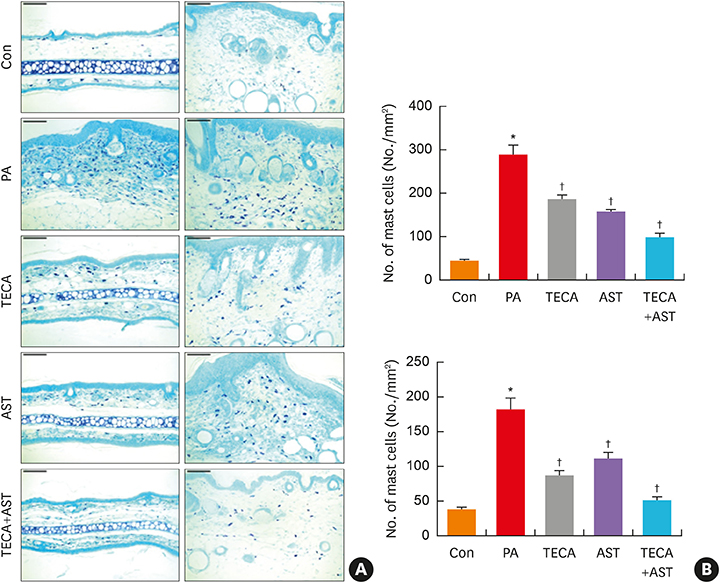
PA-induced skin morphological changes and ear thickness were significantly reduced by TECA, AST and TECA + AST treatments, but these inhibiting effects were more pronounced in the TECA + AST treatment. TECA, AST and the TECA+AST reatments inhibited the expression of iNOS and COX-2; NF-κB activity; and the release of TNF-α, IL-6 and IgE. However, the TECA+AST treatment showed additive or synergistic effects on AD.
CONCLUSIONS
Our results demonstrate that the combination of TECA and AST could be a promising therapeutic agent for AD by inhibiting NF-κB signaling.
MeSH Terms
-
Animals
Blotting, Western
Centella*
Dermatitis
Dermatitis, Atopic*
Ear
Enzyme-Linked Immunosorbent Assay
Immunoglobulin E
Immunoglobulins
Inflammation
Interleukin-6
Interleukins
Mice*
Nitric Oxide Synthase Type II
Prevalence
Skin
Tumor Necrosis Factor-alpha
Immunoglobulin E
Immunoglobulins
Interleukin-6
Interleukins
Nitric Oxide Synthase Type II
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004; 113:651–657.
Article2. Goindi S, Kumar G, Kumar N, Kaur A. Development of novel elastic vesicle-based topical formulation of cetirizine dihydrochloride for treatment of atopic dermatitis. AAPS PharmSciTech. 2013; 14:1284–1293.
Article3. Kim BE, Leung DYM. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol Res. 2018; 10:207–215.
Article4. El Hachem M, Gesualdo F, Ricci G, Diociaiuti A, Giraldi L, Ametrano O, et al. Topical corticosteroid phobia in parents of pediatric patients with atopic dermatitis: a multicentre survey. Ital J Pediatr. 2017; 43:22.
Article5. Lee CS, Lee SA, Kim YJ, Seo SJ, Lee MW. 3,4,5-tricaffeoylquinic acid inhibits tumor necrosis factor-α-stimulated production of inflammatory mediators in keratinocytes via suppression of Akt- and NF-κB-pathways. Int Immunopharmacol. 2011; 11:1715–1723.
Article6. Umezawa K, Chaicharoenpong C. Molecular design and biological activities of NF-kappaB inhibitors. Mol Cells. 2002; 14:163–167.7. Park CS, Kim TB, Moon KA, Bae YJ, Lee HR, Jang MK, et al. Chlamydophila pneumoniae enhances secretion of VEGF, TGF-beta and TIMP-1 from human bronchial epithelial cells under Th2 dominant microenvironment. Allergy Asthma Immunol Res. 2010; 2:41–47.8. Karuppagounder V, Arumugam S, Thandavarayan RA, Pitchaimani V, Sreedhar R, Afrin R, et al. Modulation of HMGB1 translocation and RAGE/NFκB cascade by quercetin treatment mitigates atopic dermatitis in NC/Nga transgenic mice. Exp Dermatol. 2015; 24:418–423.
Article9. Park JH, Yeo IJ, Han JH, Suh JW, Lee HP, Hong JT. Anti-inflammatory effect of Astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp Dermatol. 2018; 27:378–385.
Article10. Hamasaka A, Yoshioka N, Abe R, Kishino S, Umezawa K, Ozaki M, et al. Topical application of dehydroxymethylepoxyquinomicin improves allergic inflammation via NF-kappaB inhibition. J Allergy Clin Immunol. 2010; 126:400–403.11. Tanaka A, Muto S, Jung K, Itai A, Matsuda H. Topical application with a new NF-kappaB inhibitor improves atopic dermatitis in NC/NgaTnd mice. J Invest Dermatol. 2007; 127:855–863.12. Park JH, Choi JY, Son DJ, Park EK, Song MJ, Hellström M, et al. Anti-inflammatory effect of titrated extract of Centella asiatica in phthalic anhydride-induced allergic dermatitis animal model. Int J Mol Sci. 2017; 18:E738.13. Devi VK, Jain N, Valli KS. Importance of novel drug delivery systems in herbal medicines. Pharmacogn Rev. 2010; 4:27–31.14. Hong SW, Kim MR, Lee EY, Kim JH, Kim YS, Jeon SG, et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy. 2011; 66:351–359.15. Tinnell SB, Jacobs-Helber SM, Sterneck E, Sawyer ST, Conrad DH. STAT6, NF-kappaB and C/EBP in CD23 expression and IgE production. Int Immunol. 1998; 10:1529–1538.
Article16. Marquardt DL, Walker LL. Dependence of mast cell IgE-mediated cytokine production on nuclear factor-kappaB activity. J Allergy Clin Immunol. 2000; 105:500–505.17. Yoshihisa Y, Andoh T, Matsunaga K, Rehman MU, Maoka T, Shimizu T. Efficacy of astaxanthin for the treatment of atopic dermatitis in a murine model. PLoS One. 2016; 11:e0152288.
Article18. Leung DY. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J Allergy Clin Immunol. 2000; 105:860–876.
Article19. Bieber T. Atopic dermatitis. N Engl J Med. 2008; 358:1483–1494.
Article20. Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009; 21:666–678.
Article21. Jeong HJ, Koo HN, Na HJ, Kim MS, Hong SH, Eom JW, et al. Inhibition of TNF-alpha and IL-6 production by aucubin through blockade of NF-kappaB activation RBL-2H3 mast cells. Cytokine. 2002; 18:252–259.22. Matsumoto M, Yamada T, Yoshinaga SK, Boone T, Horan T, Fujita S, et al. Essential role of NF-kappa B-inducing kinase in T cell activation through the TCR/CD3 pathway. J Immunol. 2002; 169:1151–1158.23. Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011; 21:146–158.
Article24. Park JH, Kim MS, Jeong GS, Yoon J. Xanthii fructus extract inhibits TNF-α/IFN-γ-induced Th2-chemokines production via blockade of NF-κB, STAT1 and p38-MAPK activation in human epidermal keratinocytes. J Ethnopharmacol. 2015; 171:85–93.25. Tanaka A, Konno M, Muto S, Kambe N, Morii E, Nakahata T, et al. A novel NF-kappaB inhibitor, IMD-0354, suppresses neoplastic proliferation of human mast cells with constitutively activated c-kit receptors. Blood. 2005; 105:2324–2331.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antifungal Activity of Methanolic of Centella asiatica and Andrographis panicuiata
- Role of IL-10 in the Trimellitic Anhydride-induced Contact Dermatitis
- Centella asiatica enhances neurogenesis and protects neuronal cells against H2O2-induced oxidative injury
- First Report of Septoria centellae Associated with Leaf Spot of Centella asiatica in Korea
- Inhibitory Effect of Carnosol on Phthalic Anhydride-Induced Atopic Dermatitis via Inhibition of STAT3