Nutr Res Pract.
2018 Dec;12(6):469-478. 10.4162/nrp.2018.12.6.469.
Coating rice with mulberry leaves rich in deoxynojirimycin ameliorates hyperglycemia and dyslipidemia in C57BL/KsJ db/db mice
- Affiliations
-
- 1Department of Food and Nutrition, Chosun University, 309 Pilmun-daero, Dong-gu, Gwangju 61452, Korea. leejj80@chosun.ac.kr
- 2Department of Nutrition and Culinary Science, Hankyong National University, Gyeonggi 17579, Korea.
- KMID: 2448691
- DOI: http://doi.org/10.4162/nrp.2018.12.6.469
Abstract
- BACKGROUND/OBJECTIVES
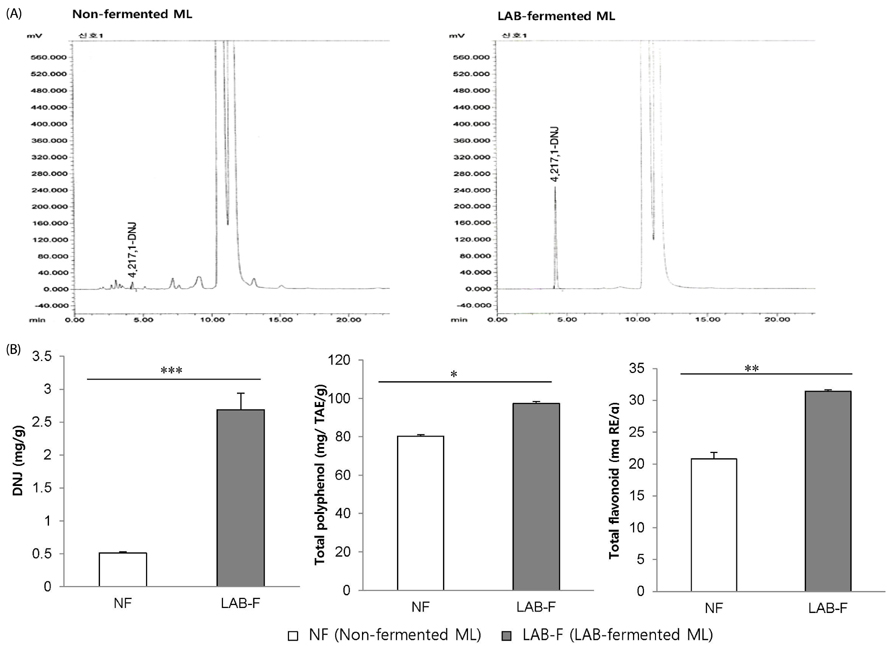
Mulberry leaf (ML) has been shown to have an inhibitory effect on α-glucosidase, and suppresses postprandial hyperglycemia, which may be related to its deoxynojirimycin (DNJ) content. This study was conducted to investigate the hypoglycemic and dyslipidemic effects of rice coated with ML rich in DNJ in a type 2 diabetes mouse model.
MATERIALS/METHODS
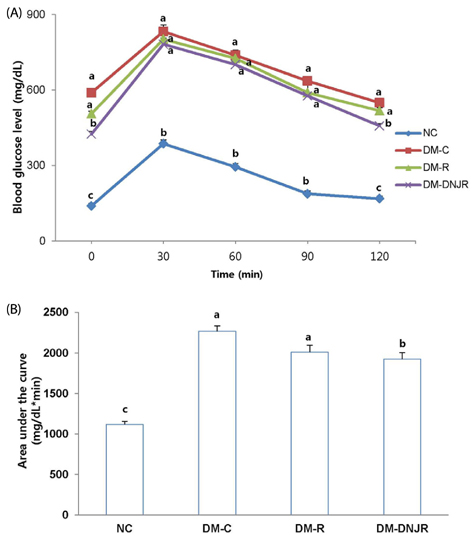
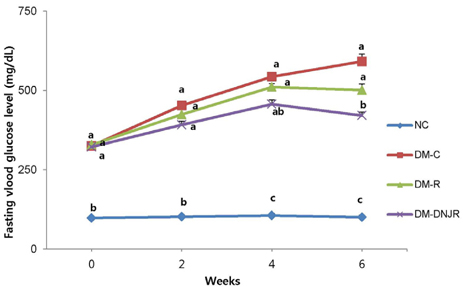
The mice were divided into four groups (n = 8 each): non-diabetic normal control (NC); diabetic control (DM-C), fed with 10% polished rice powder (DM-R); and fed with 10% polished rice powder coated with DNJ-rich ML (DM-DNJR).
RESULTS
Supplementation with DNJR for six weeks decreased levels of fasting blood glucose, plasma insulin, triglyceride, total cholesterol, and blood glycosylated hemoglobin; conversely, levels of glucagon-like peptide-1 and high-density lipoprotein-cholesterol showed an increase in the same treatment. In addition, weights of mesenteric, epididymal, and total adipose tissues decreased with DNJR supplementation, when compared with diabetic control db/db mice, while maltase, lactase, and sucrase activity in the small intestine were inhibited. The anti-diabetic effects were marginally greater in the DM-DNJR group than in the DM-R group.
CONCLUSIONS
These results suggest that rice coated with ML rich in DNJ can reduce hyperglycemia and hyperlipidemia in db/db mice, and may prove useful for individuals with diabetes.
Keyword
MeSH Terms
-
Animals
Blood Glucose
Cholesterol
Diabetes Mellitus
Dyslipidemias*
Fasting
Glucagon-Like Peptide 1
Hemoglobin A, Glycosylated
Hyperglycemia*
Hyperlipidemias
Hypoglycemia
Insulin
Intestine, Small
Lactase
Mice*
Morus*
Plasma
Sucrase
Triglycerides
Weights and Measures
Blood Glucose
Cholesterol
Glucagon-Like Peptide 1
Insulin
Lactase
Sucrase
Figure
Reference
-
1. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988; 37:1595–1607.
Article2. Hayden JM, Reaven PD. Cardiovascular disease in diabetes mellitus type 2: a potential role for novel cardiovascular risk factors. Curr Opin Lipidol. 2000; 11:519–528.
Article3. Bailey CJ. Insulin resistance and antidiabetic drugs. Biochem Pharmacol. 1999; 58:1511–1520.
Article4. Hu XQ, Thakur K, Chen GH, Hu F, Zhang JG, Zhang HB, Wei ZJ. Metabolic effect of 1-deoxynojirimycin from mulberry leaves on db/db diabetic mice using liquid chromatography-mass spectrometry based metabolomics. J Agric Food Chem. 2017; 65:4658–4667.
Article5. Han CK, Kim SS, Choi SY, Park JH, Lee BH. Effects of rice added with mulberry leaves and fruit on blood glucose, body fat and serum lipid levels in rats. J Korean Soc Food Sci Nutr. 2009; 38:1336–1341.
Article6. Li YG, Ji DF, Zhong S, Lv ZQ, Lin TB, Chen S, Hu GY. Hybrid of 1-deoxynojirimycin and polysaccharide from mulberry leaves treat diabetes mellitus by activating PDX-1/insulin-1 signaling pathway and regulating the expression of glucokinase, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in alloxan-induced diabetic mice. J Ethnopharmacol. 2011; 134:961–970.
Article7. Li YG, Ji DF, Zhong S, Lv ZQ, Lin TB. Cooperative anti-diabetic effects of deoxynojirimycin-polysaccharide by inhibiting glucose absorption and modulating glucose metabolism in streptozotocin-induced diabetic mice. PLoS One. 2013; 8:e65892.
Article8. Liu Q, Li X, Li C, Zheng Y, Wang F, Li H, Peng G. 1-Deoxynojirimycin alleviates liver injury and improves hepatic glucose metabolism in db/db mice. Molecules. 2016; 21:279.
Article9. Riche DM, Riche KD, East HE, Barrett EK, May WL. Impact of mulberry leaf extract on type 2 diabetes (Mul-DM): a randomized, placebo-controlled pilot study. Complement Ther Med. 2017; 32:105–108.
Article10. Józefczuk J, Malikowska K, Glapa A, Stawińska-Witoszyńska B, Nowak JK, Bajerska J, Lisowska A, Walkowiak J. Mulberry leaf extract decreases digestion and absorption of starch in healthy subjects-A randomized, placebo-controlled, crossover study. Adv Med Sci. 2017; 62:302–306.
Article11. Ryu IH, Kwon TO. Functional quality characteristics of extracts by sugar-leaching and lactic acid fermentation of mulberry leaves (Morus alba L.). J Sericult Entomol Sci. 2013; 51:164–172.
Article12. Sharma RD, Rukmini C. Rice bran oil and hypocholesterolemia in rats. Lipids. 1986; 21:715–717.
Article13. Ha TY, Ko SN, Lee SM, Kim HR, Chung SH, Kim SR, Yoon HH, Kim IH. Changes in nutraceutical lipid components of rice at different degree of milling. Eur J Lipid Sci Technol. 2006; 108:175–181.
Article14. Kahlon TS, Saunders RM, Sayre RN, Chow FI, Chiu MM, Betschart AA. Cholesterol-lowering effects of rice bran and rice bran oil fractions in hypercholesterolemic hamsters. Cereal Chem. 1992; 69:485–489.15. Crapo PA, Insel J, Sperling M, Kolterman OG. Comparison of serum glucose, insulin, and glucagon responses to different types of complex carbohydrate in noninsulin-dependent diabetic patients. Am J Clin Nutr. 1981; 34:184–190.
Article16. Jiraratsatit J, Mangklabruks A, Keoplung M, Matayabun S, Chumsilp L. Glycemic effects of rice and glutinous rice on treated-type II diabetic subjects. J Med Assoc Thai. 1987; 70:401–409.17. Ha TY. Health functional properties of rice. Food Ind Nutr. 2008; 13:22–26.18. Stead DA, Richards RM. Sensitive fluorimetric determination of gentamicin sulfate in biological matrices using solid-phase extraction, pre-column derivatization with 9-fluorenylmethyl chloroformate and reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1996; 675:295–302.
Article19. Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002; 37:153–161.
Article20. Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002; 10:178–182.
Article21. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951.
Article22. Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003; 28:916–931.
Article23. Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968; 22:99–107.
Article24. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.
Article25. Rosenfeld L. Lipoprotein analysis. Early methods in the diagnosis of atherosclerosis. Arch Pathol Lab Med. 1989; 113:1101–1110.26. Katina K, Liukkonen KH, Kaukovirta-Norja A, Adlercreutz H, Heinonen SM, Lampi AM, Pihlava JM, Poutanen K. Fermentation-induced changes in the nutritional value of native or germinated rye. J Cereal Sci. 2007; 46:348–355.
Article27. Chae JY, Lee JY, Hoang IS, Whangbo D, Choi PW, Lee WC, Kim JW, Kim SY, Choi SW, Rhee SJ. Analysis of functional components of leaf of different mulberry cultivars. J Korean Soc Food Sci Nutr. 2003; 32:15–21.28. Lee SM, Bustamante S, Flores C, Bezerra J, Goda T, Koldovský O. Chronic effects of an alpha-glucosidase inhibitor (Bay o 1248) on intestinal disaccharidase activity in normal and diabetic mice. J Pharmacol Exp Ther. 1987; 240:132–137.29. Orland MJ, Permutt MA. Quantitative analysis of pancreatic proinsulin mRNA in genetically diabetic (db/db) mice. Diabetes. 1987; 36:341–347.
Article30. Park KS, Lee DE, Sung JH, Chung SH. Comparisons of antidiabetic effect of Panax ginseng on MLD STZ-induced diabetic rats in terms of time of administration. J Ginseng Res. 2002; 26:191–195.
Article31. Park BH, Shin JW, Lee SI, Kim SD. The effects of Cudrania tricupidata tea leaves on the blood glucose and serum lipids profiles of streptozotocin-induced hyperglycemic rats. J East Asian Soc Diet Life. 2008; 18:516–523.32. Lee SG, Han KS, Jeong SG, Oh MH, Jang AR, Kim DH, Bae IH, Ham JS. A study on the sensory characteristic of yogurt and antimicrobial activity of Lactobacillus plantarum LHC52 isolated from kimchi. J Korean Soc Food Sci Anim Resour. 2010; 30:328–335.
Article33. Goldberg RB. Lipid disorders in diabetes. Diabetes Care. 1981; 4:561–572.
Article34. Matsumoto N, Ishigaki F, Ishigaki A, Iwashina H, Hara Y. Reduction of blood glucose levels by tea catechin. Biosci Biotechnol Biochem. 1993; 57:525–527.
Article35. Kim SY, Ryu KS, Lee WC, Ku HO, Lee HS, Lee KR. Hypoglycemic effect of mulberry leaves with anaerobic treatment in alloxan-induced diabetic mice. Korean J Pharmacogn. 1999; 30:123–129.36. Yang JH, Han JS. Effect of mulberry leaf extract supplement on blood glucose, glycated hemoglobin and serum lipids in type II diabetic patients. J Korean Soc Food Sci Nutr. 2006; 35:549–556.
Article37. Asia Pacific Cohort Studies Collaboration. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004; 27:2836–2842.38. Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–986.
Article39. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–853.40. Janle EM, Portocarrero C, Zhu Y, Zhou Q. Effect of long-term oral administration of green tea extract on weight gain and glucose tolerance in Zucker diabetic (ZDF) rats. J Herb Pharmacother. 2005; 5:55–65.
Article41. Kannel WB, Wilson PW, Zhang TJ. The epidemiology of impaired glucose tolerance and hypertension. Am Heart J. 1991; 121:1268–1273.
Article42. DeFronzo RA. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia. 1981; 21:165–171.43. Chung SH, Yu JH, Kim EJ, Ryu KS. Blood glucose lowering effect of silkworm. Bull K H Pharma Sci. 1996; 24:95–100.44. Trümper K, Trümper A, Trusheim H, Arnold R, Göke B, Hörsch D. Integrative mitogenic role of protein kinase B/Akt in beta-cells. Ann N Y Acad Sci. 2000; 921:242–250.45. Rahbar S. An abnormal hemoglobin in red cells of diabetics. Clin Chim Acta. 1968; 22:296–298.
Article46. Park HR, Cho JS. Effects of a natural medicinal multi-plant extract on blood glucose, insulin levels, and serum malondialdehyde concentrations in streptozotocin-induced diabetic rats. J East Asian Soc Diet Life. 2007; 17:205–212.47. Khalafallah A, Phuah E, Al-Barazan AM, Nikakis I, Radford A, Clarkson W, Trevett C, Brain T, Gebski V, Corbould A. Glycosylated haemoglobin for screening and diagnosis of gestational diabetes mellitus. BMJ Open. 2016; 6:e011059.
Article48. Lee JS, Lee JS, Yang CB, Shin HK. Blood glucose response to some cereals and determination of their glycemic index to rice as the standard food. Korean J Nutr. 1997; 30:1170–1179.49. Kimura T, Nakagawa K, Kubota H, Kojima Y, Goto Y, Yamagishi K, Oita S, Oikawa S, Miyazawa T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J Agric Food Chem. 2007; 55:5869–5874.
Article50. Kennedy DL, Piper JM, Baum C. Trends in use of oral hypoglycemic agents 1964-1986. Diabetes Care. 1988; 11:558–562.
Article51. Fuliang HU, Hepburn HR, Xuan H, Chen M, Daya S, Radloff SE. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol Res. 2005; 51:147–152.
Article52. Park CH, Lee GD, Kim JO, Kim KS, Lee WY, Hong JH. Effect of Bulnesia sarmienti water extracts on lipid metabolism in neonatally streptozotocin-induced diabetic rats. J Life Sci. 2008; 8:999–1004.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sargassum coreanum extract alleviates hyperglycemia and improves insulin resistance in db/db diabetic mice
- Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic beta cell mass in C57BL/KsJ-db/db mice
- Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice
- Gynura procumbens extract improves insulin sensitivity and suppresses hepatic gluconeogenesis in C57BL/KsJ-db/db mice
- Daraesoon (shoot of hardy kiwi) mitigates hyperglycemia in db/db mice by alleviating insulin resistance and inflammation