Anesth Pain Med.
2019 Apr;14(2):117-122. 10.17085/apm.2019.14.2.117.
The potential risks of sugammadex
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Busan Paik Hospital, Busan, Korea. 2wonjin@naver.com
- 2Paik Institute for Clinical Research, Inje University College of Medicine, Busan, Korea.
- KMID: 2447958
- DOI: http://doi.org/10.17085/apm.2019.14.2.117
Abstract
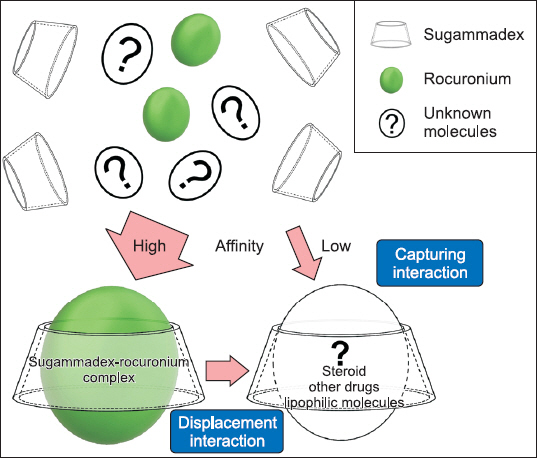
- Sugammadex provides fast and safe recovery from neuromuscular blockade without causing major adverse effects, and its clinical use is increasing. However, there are some reports on the potential risks of sugammadex, such as severe bradycardia, interactions with steroids, coagulopathy, and neuronal damage. Although these potential risks are not clearly proven, they are considered to be dose-dependent and occur more frequently with the free-form of sugammadex. Until further pieces of evidence are accumulated, it is prudent to be aware of these potential risks and avoid an overdose of sugammadex.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Sugammadex for our little ones: a brief narrative review
Soomin Lee, Woosuk Chung
Anesth Pain Med. 2024;19(4):269-279. doi: 10.17085/apm.24092.
Reference
-
1. Carron M, Zarantonello F, Tellaroli P, Ori C. Efficacy and safety of sugammadex compared to neostigmine for reversal of neuromuscular blockade: a meta-analysis of randomized controlled trials. J Clin Anesth. 2016; 35:1–12. DOI: 10.1016/j.jclinane.2016.06.018. PMID: 27871504.2. Hristovska AM, Duch P, Allingstrup M, Afshari A. The comparative efficacy and safety of sugammadex and neostigmine in reversing neuromuscular blockade in adults. A Cochrane systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 2018; 73:631–41. DOI: 10.1111/anae.14160. PMID: 29280475.3. Abad-Gurumeta A, Ripollés-Melchor J, Casans-Francés R, Espinosa A, Martínez-Hurtado E, Fernández-Pérez C, et al. A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade. Anaesthesia. 2015; 70:1441–52. DOI: 10.1111/anae.13277. PMID: 26558858.4. Won YJ, Lim BG, Lee DK, Kim H, Kong MH, Lee IO. Sugammadex for reversal of rocuronium-induced neuromuscular blockade in pediatric patients: a systematic review and meta-analysis. Medicine (Baltimore). 2016; 95:e4678. DOI: 10.1097/MD.0000000000004678. PMID: 27559972. PMCID: PMC5400339.5. Liu G, Wang R, Yan Y, Fan L, Xue J, Wang T. The efficacy and safety of sugammadex for reversing postoperative residual neuromuscular blockade in pediatric patients: a systematic review. Sci Rep. 2017; 7:5724. DOI: 10.1038/s41598-017-06159-2. PMID: 28720838. PMCID: PMC5515941.6. Choi S, Jung M, Kim KM, Lee S, Yang HS, Lee J. A survey of current concepts and practices related to use of neuromuscular blockers with antagonists and neuromuscular monitoring among Korean anesthesiologists. Anesth Pain Med. 2018; 13:47–52. DOI: 10.17085/apm.2018.13.1.47.7. Karalapillai D, Kaufman M, Weinberg L. Sugammadex. Crit Care Resusc. 2013; 15:57–62.8. Mirakhur RK. Sugammadex in clinical practice. Anaesthesia. 2009; 64(Suppl 1):45–54. DOI: 10.1111/j.1365-2044.2008.05870.x. PMID: 19222431.9. Zwiers A, van den Heuvel M, Smeets J, Rutherford S. Assessment of the potential for displacement interactions with sugammadex: a pharmacokinetic-pharmacodynamic modelling approach. Clin Drug Investig. 2011; 31:101–11. DOI: 10.1007/BF03256937. PMID: 21067251.10. Godai K, Hasegawa-Moriyama M, Kuniyoshi T, Kakoi T, Ikoma K, Isowaki S, et al. Three cases of suspected sugammadex-induced hypersensitivity reactions. Br J Anaesth. 2012; 109:216–8. DOI: 10.1093/bja/aes137. PMID: 22617091.11. Menéndez-Ozcoidi L, Ortiz-Gómez JR, Olaguibel-Ribero JM, Salvador-Bravo MJ. Allergy to low dose sugammadex. Anaesthesia. 2011; 66:217–9. DOI: 10.1111/j.1365-2044.2010.06611.x. PMID: 21320089.12. Dahl V, Pendeville PE, Hollmann MW, Heier T, Abels EA, Blob- ner M. Safety and efficacy of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in cardiac patients undergoing noncardiac surgery. Eur J Anaesthesiol. 2009; 26:874–84. DOI: 10.1097/EJA.0b013e32832c605b. PMID: 19455040.13. Hunter JM, Naguib M. Sugammadex-induced bradycardia and asystole: how great is the risk? Br J Anaesth. 2018; 121:8–12. DOI: 10.1016/j.bja.2018.03.003. PMID: 29935599.14. Miyazaki Y, Sunaga H, Kida K, Hobo S, Inoue N, Muto M, et al. Incidence of anaphylaxis associated with sugammadex. Anesth Analg. 2018; 126:1505–8. DOI: 10.1213/ANE.0000000000002562. PMID: 29064876.15. Bhavani SS. Severe bradycardia and asystole after sugammadex. Br J Anaesth. 2018; 121:95–6. DOI: 10.1016/j.bja.2018.02.036. PMID: 29935601.16. Cammu G, De Kam PJ, Demeyer I, Decoopman M, Peeters PA, Smeets JM, et al. Safety and tolerability of single intravenous doses of sugammadex administered simultaneously with rocuronium or vecuronium in healthy volunteers. Br J Anaesth. 2008; 100:373–9. DOI: 10.1093/bja/aem402. PMID: 18238834.17. Kokki M, Ali M, Turunen M, Kokki H. Suspected unexpected adverse effect of sugammadex: hypotension. Eur J Clin Pharmacol. 2012; 68:899–900. DOI: 10.1007/s00228-011-1196-z. PMID: 22205274.18. Pühringer FK, Rex C, Sielenkämper AW, Claudius C, Larsen PB, Prins ME, et al. Reversal of profound, high-dose rocuronium-induced neuromuscular blockade by sugammadex at two different time points: an international, multicenter, randomized, dosefinding, safety assessor-blinded, phase II trial. Anesthesiology. 2008; 109:188–97. DOI: 10.1097/ALN.0b013e31817f5bc7. PMID: 18648227.19. Sanoja IA, Toth KS. Profound bradycardia and cardiac arrest after sugammadex administration in a previously healthy patient: a case report. A A Pract. 2019; 12:22–4. DOI: 10.1213/XAA.0000000000000834. PMID: 30004912.20. Rezonja K, Sostaric M, Vidmar G, Mars T. Dexamethasone produces dose-dependent inhibition of sugammadex reversal in in vitro innervated primary human muscle cells. Anesth Analg. 2014; 118:755–63. DOI: 10.1213/ANE.0000000000000108. PMID: 24651229.21. Buonanno P, Laiola A, Palumbo C, Spinelli G, Servillo G, Di Minno RM, et al. Dexamethasone does not inhibit sugammadex reversal after rocuronium-induced neuromuscular block. Anesth Analg. 2016; 122:1826–30. DOI: 10.1213/ANE.0000000000001294. PMID: 27028777.22. Gulec E, Biricik E, Turktan M, Hatipoglu Z, Unlugenc H. The effect of intravenous dexamethasone on sugammadex reversal time in children undergoing adenotonsillectomy. Anesth Analg. 2016; 122:1147–52. DOI: 10.1213/ANE.0000000000001142. PMID: 26771267.23. Rezonja K, Mars T, Jerin A, Kozelj G, Pozar-Lukanovic N, Sostaric M. Dexamethasone does not diminish sugammadex reversal of neuromuscular block - clinical study in surgical patients undergoing general anesthesia. BMC Anesthesiol. 2016; 16:101. DOI: 10.1186/s12871-016-0254-6. PMID: 27765010. PMCID: PMC5073416.24. Ozer AB, Bolat E, Erhan OL, Kilinc M, Demirel I, Toprak GC. Sugammadex improves neuromuscular function in patients receiving perioperative steroids. Niger J Clin Pract. 2018; 21:139–42. DOI: 10.4103/njcp.njcp_322_16. PMID: 29465045.25. Gunduz Gul G, Ozer AB, Demirel I, Aksu A, Erhan OL. The effect of sugammadex on steroid hormones: a randomized clinical study. J Clin Anesth. 2016; 34:62–7. DOI: 10.1016/j.jclinane.2016.03.039. PMID: 27687347.26. Dirkmann D, Britten MW, Pauling H, Weidle J, Volbracht L, Gör-linger K, et al. Anticoagulant effect of sugammadex: just an in vitro artifact. Anesthesiology. 2016; 124:1277–85. DOI: 10.1097/ALN.0000000000001076. PMID: 26950705.27. Lee IO, Kim YS, Chang HW, Kim H, Lim BG, Lee M. In vitro investigation of the effects of exogenous sugammadex on coagulation in orthopedic surgical patients. BMC Anesthesiol. 2018; 18:56. DOI: 10.1186/s12871-018-0519-3. PMID: 29793426. PMCID: PMC5968558.28. Carron M, Bertini D, Prandini T, Fanton F, Foletto M, Ori C, et al. Effect of sugammadex on coagulation as detected by rotational thromboelastometry in morbidly obese patients. Minerva Anestesiol. 2018; 84:178–88. DOI: 10.23736/S0375-9393.17.11856-0. PMID: 28714298.29. Taş N, Korkmaz H, Yağan Ö, Korkmaz M. Effect of sugammadex on postoperative bleeding and coagulation parameters after septoplasty: a randomized prospective study. Med Sci Monit. 2015; 21:2382–6. DOI: 10.12659/MSM.894971. PMID: 26271275. PMCID: PMC4540056.30. Moon YJ, Kim SH, Kim JW, Lee YK, Jun IG, Hwang GS. Comparison of postoperative coagulation profiles and outcome for sugammadex versus pyridostigmine in 992 living donors after living-donor hepatectomy. Medicine (Baltimore). 2018; 97:e0129. DOI: 10.1097/MD.0000000000010129. PMID: 29538210. PMCID: PMC5882409.31. Palanca JM, Aguirre-Rueda D, Granell MV, Aldasoro M, Garcia A, Iradi A, et al. Sugammadex, a neuromuscular blockade reversal agent, causes neuronal apoptosis in primary cultures. Int J Med Sci. 2013; 10:1278–85. DOI: 10.7150/ijms.6254. PMID: 23983586. PMCID: PMC3752716.32. Aldasoro M, Jorda A, Aldasoro C, Marchio P, Guerra-Ojeda S, Gimeno-Raga M, et al. Neuronal effects of sugammadex in combination with rocuronium or vecuronium. Int J Med Sci. 2017; 14:224–30. DOI: 10.7150/ijms.17545. PMID: 28367082. PMCID: PMC5370284.33. Ozbilgin S, Yılmaz O, Ergur BU, Hancı V, Ozbal S, Yurtlu S, et al. Effectiveness of sugammadex for cerebral ischemia/reperfusion injury. Kaohsiung J Med Sci. 2016; 32:292–301. DOI: 10.1016/j.kjms.2016.05.002. PMID: 27377841.34. Batistaki C, Tentes P, Deligiannidi P, Karakosta A, Florou P, Kos-topanagiotou G. Residual neuromuscular blockade in a real life clinical setting: correlation with sugammadex or neostigmine administration. Minerva Anestesiol. 2016; 82:550–8. PMID: 26394365.35. Pongrácz A, Szatmári S, Nemes R, Fülesdi B, Tassonyi E. Reversal of neuromuscular blockade with sugammadex at the reappearance of four twitches to train-of-four stimulation. Anesthesiology. 2013; 119:36–42. DOI: 10.1097/ALN.0b013e318297ce95. PMID: 23665915.36. Loupec T, Frasca D, Rousseau N, Faure JP, Mimoz O, Debaene B. Appropriate dosing of sugammadex to reverse deep rocuronium-induced neuromuscular blockade in morbidly obese patients. Anaesthesia. 2016; 71:265–72. DOI: 10.1111/anae.13344. PMID: 26685122.37. Abd El-Rahman AM, Othman AH, El Sherif FA, Mostafa MF, Taha O. Comparison of three different doses sugammadex based on ideal body weight for reversal of moderate rocuronium-induced neuromuscular blockade in laparoscopic bariatric surgery. Minerva Anestesiol. 2017; 83:138–44. DOI: 10.23736/S0375-9393.16.11349-5. PMID: 27575450.38. Badaoui R, Cabaret A, Alami Y, Zogheib E, Popov I, Lorne E, et al. Reversal of neuromuscular blockade by sugammadex in laparoscopic bariatric surgery: in support of dose reduction. Anaesth Crit Care Pain Med. 2016; 35:25–9. DOI: 10.1016/j.accpm.2015.09.003. PMID: 26597732.39. Monk TG, Rietbergen H, Woo T, Fennema H. Use of sugammadex in patients with obesity: a pooled analysis. Am J Ther. 2017; 24:e507–16. DOI: 10.1097/MJT.0000000000000305. PMID: 26398716.40. Carron M, Zarantonello F, Ori C. Dose of sugammadex in morbidly obese patients. Anaesthesia. 2016; 71:730–1. DOI: 10.1111/anae.13473. PMID: 27159001.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anaphylaxis induced by sugammadex and sugammadex-rocuronium complex -a case report-
- What we need to know and do on sugammadex usage in pregnant and lactating women and those on hormonal contraceptives
- Advantages and pitfalls of clinical application of sugammadex
- In the hour of Sugammadex
- Sugammadex-induced bronchospasm: a case report