Endocrinol Metab.
2018 Sep;33(3):380-386. 10.3803/EnM.2018.33.3.380.
Genetic Analysis of CLCN7 in an Old Female Patient with Type II Autosomal Dominant Osteopetrosis
- Affiliations
-
- 1Department of Laboratory Medicine, Chungnam National University College of Medicine, Daejeon, Korea.
- 2Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea. jmpbooks@cnuh.co.kr
- 3Research Center for Endocrine and Metabolic Diseases, Chungnam National University College of Medicine, Daejeon, Korea.
- KMID: 2447028
- DOI: http://doi.org/10.3803/EnM.2018.33.3.380
Abstract
- BACKGROUND
Type II autosomal dominant osteopetrosis (ADO II) is a rare genetically heterogeneous disorder characterized by osteosclerosis and increased bone mass, predominantly involving spine, pelvis, and skull. It is closely related to functional defect of osteoclasts caused by chloride voltage-gated channel 7 (CLCN7) gene mutations. In this study, we aimed to identify the pathogenic mutation in a Korean patient with ADO II using whole exome sequencing.
METHODS
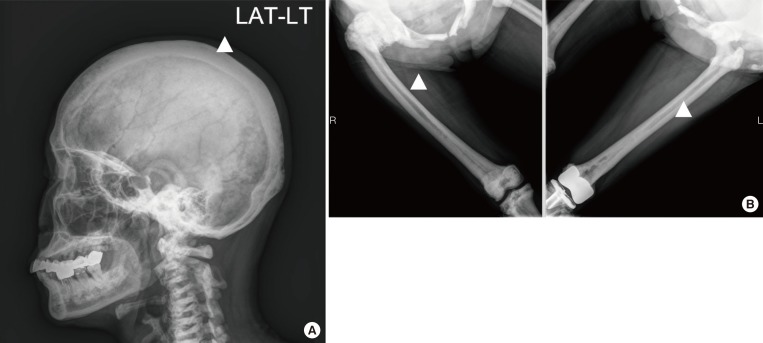
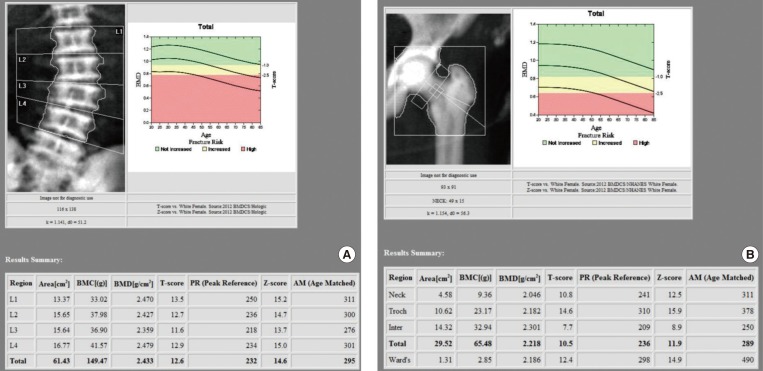
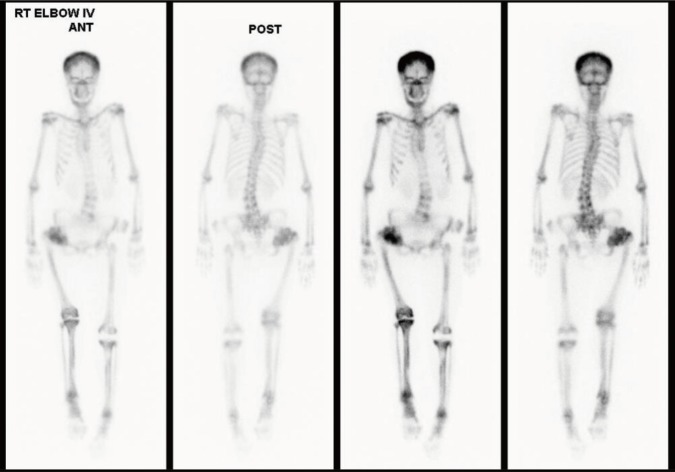
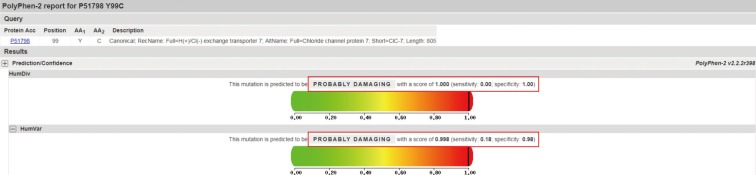
We evaluated the clinical, biochemical, and radiographic analysis of a 68-year-old woman with ADO II. We also performed whole exome sequencing to identify pathogenic mutation of a rare genetic disorder of the skeleton. Moreover, a polymorphism phenotyping program, Polymorphism Phenotyping v2 (PolyPhen-2), was used to assess the effect of the identified mutation on protein function.
RESULTS
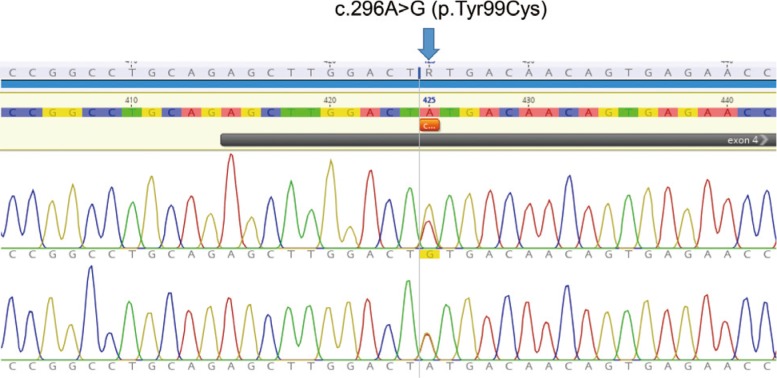
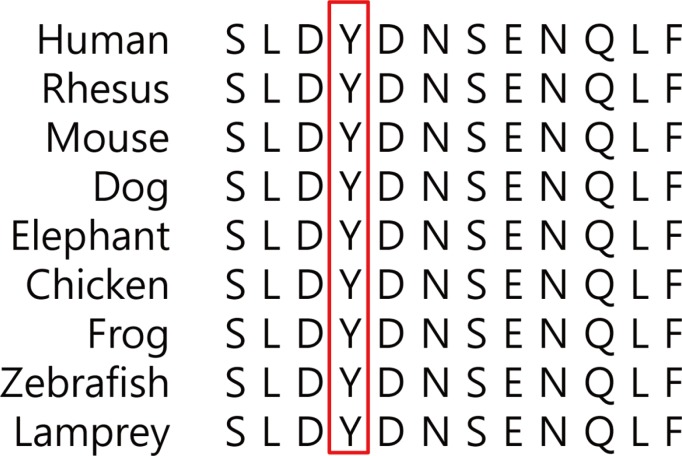
Whole exome sequencing using peripheral leukocytes revealed a heterozygous c.296A>G missense mutation in the CLCN7 gene. The mutation was also confirmed using Sanger sequencing. The mutation c.296A>G was regarded to have a pathogenic effect by PolyPhen-2 software.
CONCLUSION
We detect a heterozygous mutation in CLCN7 gene of a patient with ADO II, which is the first report in Korea. Our present findings suggest that symptoms and signs of ADO II patient having a c.296A>G mutation in CLCN7 may appear at a very late age. The present study would also enrich the database of CLCN7 mutations and improve our understanding of ADO II.
MeSH Terms
Figure
Reference
-
1. Bollerslev J, Henriksen K, Nielsen MF, Brixen K, Van Hul W. Autosomal dominant osteopetrosis revisited: lessons from recent studies. Eur J Endocrinol. 2013; 169:R39–R57. PMID: 23744590.3. Coudert AE, de Vernejoul MC, Muraca M, Del Fattore A. Osteopetrosis and its relevance for the discovery of new functions associated with the skeleton. Int J Endocrinol. 2015; 2015:372156. PMID: 25873953.
Article4. Benichou OD, Laredo JD, de Vernejoul MC. Type II autosomal dominant osteopetrosis (Albers-Schonberg disease): clinical and radiological manifestations in 42 patients. Bone. 2000; 26:87–93. PMID: 10617161.5. Del Fattore A, Peruzzi B, Rucci N, Recchia I, Cappariello A, Longo M, et al. Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: implications for diagnosis and treatment. J Med Genet. 2006; 43:315–325. PMID: 16118345.
Article6. Kasper D, Planells-Cases R, Fuhrmann JC, Scheel O, Zeitz O, Ruether K, et al. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 2005; 24:1079–1091. PMID: 15706348.
Article7. Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002; 30:3894–3900. PMID: 12202775.
Article8. Senel K, Ugur M, Erdal A, Ozdemir H. Type II autosomal dominant osteopetrosis. Rheumatol Int. 2002; 22:116–118. PMID: 12111087.
Article9. Waguespack SG, Hui SL, Dimeglio LA, Econs MJ. Autosomal dominant osteopetrosis: clinical severity and natural history of 94 subjects with a chloride channel 7 gene mutation. J Clin Endocrinol Metab. 2007; 92:771–778. PMID: 17164308.
Article10. Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000; 113(Pt 3):377–381. PMID: 10639325.
Article11. Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001; 104:205–215. PMID: 11207362.
Article12. Cleiren E, Benichou O, Van Hul E, Gram J, Bollerslev J, Singer FR, et al. Albers-Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet. 2001; 10:2861–2867. PMID: 11741829.
Article13. Schaller S, Henriksen K, Sveigaard C, Heegaard AM, Helix N, Stahlhut M, et al. The chloride channel inhibitor NS3736 [corrected] prevents bone resorption in ovariectomized rats without changing bone formation. J Bone Miner Res. 2004; 19:1144–1153. PMID: 15176998.14. Mullard A. Merck &Co. drops osteoporosis drug odanacatib. Nat Rev Drug Discov. 2016; 15:669.15. Benichou O, Cleiren E, Gram J, Bollerslev J, de Vernejoul MC, Van Hul W. Mapping of autosomal dominant osteopetrosis type II (Albers-Schonberg disease) to chromosome 16p13.3. Am J Hum Genet. 2001; 69:647–654. PMID: 11468688.16. Pang Q, Chi Y, Zhao Z, Xing X, Li M, Wang O, et al. Novel mutations of CLCN7 cause autosomal dominant osteopetrosis type II (ADO-II) and intermediate autosomal recessive osteopetrosis (IARO) in Chinese patients. Osteoporos Int. 2016; 27:1047–1055. PMID: 26395888.
Article17. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424. PMID: 25741868.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autosomal Dominant Type I Osteopetrosis Is Related with Iatrogenic Fractures in Arthroplasty
- Osteopetrosis combined with Subtrochanteric Fracture of Femur: A Case Report
- A Case of Spinocerebellar Ataxia Type 7 with Torticollis
- A Case of Cavernous Sinus Thrombophlebitis and Meningitis as a Complication in Osteopetrosis
- Autosomal Dominant Cerebellar Ataxia Type II Associated with Optic Atrophy