Nutr Res Pract.
2018 Oct;12(5):378-386. 10.4162/nrp.2018.12.5.378.
Corni Fructus attenuates testosterone-induced benign prostatic hyperplasia by suppressing 5α-reductase and androgen receptor expression in rats
- Affiliations
-
- 1Anti-Aging Research Center, Dongeui University, Busan 47340, Korea. choiyh@deu.ac.kr
- 2Open Laboratory for Muscular and Skeletal Disease and Department of Biochemistry, Dongeui University College of Korean Medicine, 42 San, Yangjungdong, Busan 47227, Korea.
- 3Department of Anatomy, Kosin University College of Medicine, Busan 49267, Korea.
- 4Gurye Sansooyu Farming Association Corporation, Jeonnam 57602, Korea.
- 5Gurye-gun Agricultural Center, Jeonnam 57660, Korea.
- 6Department of Anatomy, Dongeui University College of Korean Medicine, Busan 47227, Korea.
- 7Laboratory of Immunobiology, Department of Marine Life Sciences, Jeju National University, Jeju 63243, Korea.
- 8Department of Molecular Biology, College of Natural Sciences, Dongeui University, Busan 47340, Korea.
- KMID: 2446997
- DOI: http://doi.org/10.4162/nrp.2018.12.5.378
Abstract
- BACKGROUND/OBJECTIVES
Benign prostatic hypertrophy (BPH) is a major cause of abnormal overgrowth of the prostate mainly in the elderly. Corni Fructus has been reported to be effective in the prevention and treatment of various diseases because of its strong antioxidant effect, but its efficacy against BPH is not yet known. This study was designed to evaluate the therapeutic efficacy of Corni Fructus water extract (CF) in testosterone-induced BPH rats.
MATERIALS/METHODS
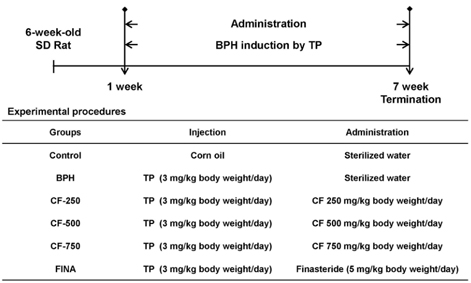
To induce BPH, rats were intraperitoneal injected with testosterone propionate (TP). Rats in the treatment group were orally administered with CF with TP injection, and finasteride, which is a selective inhibitor of 5α-reductase type 2, was used as a positive control.
RESULTS
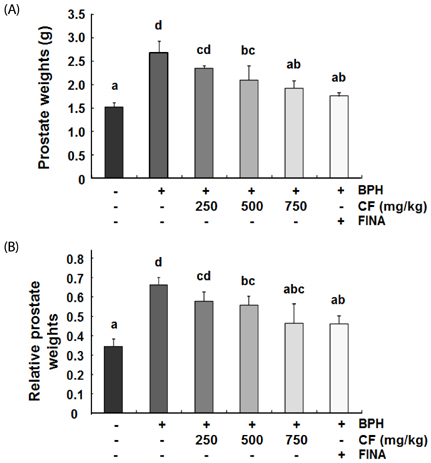
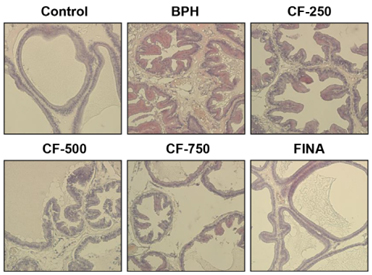
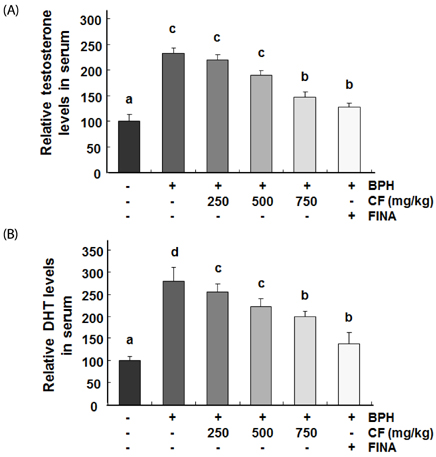
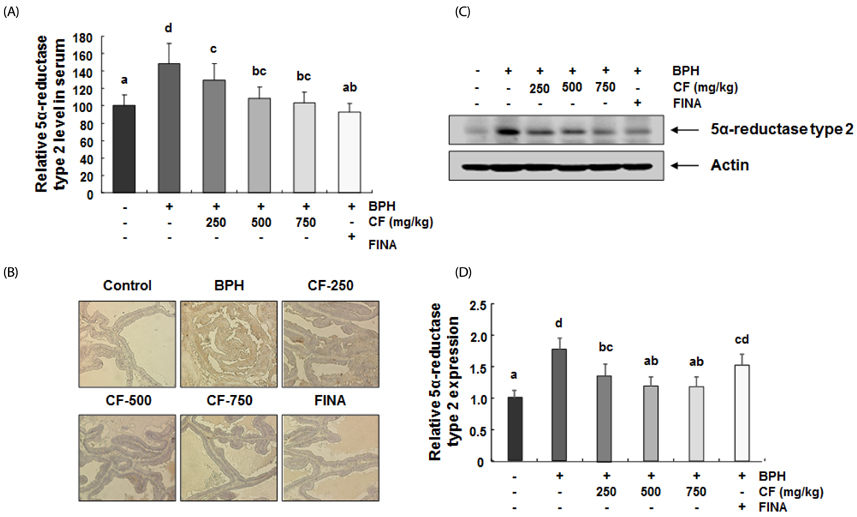
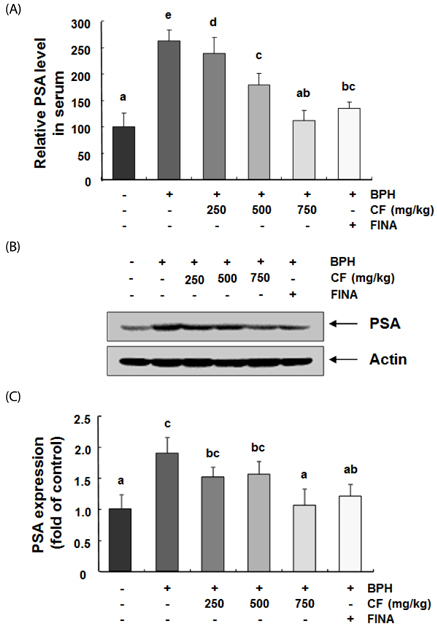
Our results showed that the increased prostate weight and histopathological changes in BPH model rats were suppressed by CF treatment. CF, similar to the finasteride-treated group, decreased the levels of testosterone and dihydrotestosterone by TP treatment in the serum, and it also reduced 5α-reductase expression and concentration in prostate tissue and serum, respectively. In addition, CF significantly blocked the expression of the androgen receptor (AR), AR co-activators, and proliferating cell nuclear antigen in BPH rats, and this blocking was associated with a decrease in prostate-specific antigen levels in serum and prostate tissue.
CONCLUSIONS
These results suggest that CF may weaken the BPH status through the inactivation of at least 5α-reductase and AR activity and may be useful for the clinical treatment of BPH.
Keyword
MeSH Terms
-
Aged
Animals
Antioxidants
Cornus*
Dihydrotestosterone
Finasteride
Humans
Proliferating Cell Nuclear Antigen
Prostate
Prostate-Specific Antigen
Prostatic Hyperplasia*
Rats*
Receptors, Androgen*
Testosterone
Testosterone Propionate
Water
Antioxidants
Dihydrotestosterone
Finasteride
Proliferating Cell Nuclear Antigen
Prostate-Specific Antigen
Receptors, Androgen
Testosterone
Testosterone Propionate
Water
Figure
Reference
-
1. Cornu JN, Ahyai S, Bachmann A, de la Rosette J, Gilling P, Gratzke C, McVary K, Novara G, Woo H, Madersbacher S. A systematic review and meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic obstruction: an update. Eur Urol. 2015; 67:1066–1096.
Article2. Pyo JS, Cho WJ. Systematic review and meta-analysis of prostatic artery embolisation for lower urinary tract symptoms related to benign prostatic hyperplasia. Clin Radiol. 2017; 72:16–22.
Article3. Aaron L, Franco OE, Hayward SW. Review of prostate anatomy and embryology and the etiology of benign prostatic hyperplasia. Urol Clin North Am. 2016; 43:279–288.
Article4. Strand DW, Costa DN, Francis F, Ricke WA, Roehrborn CG. Targeting phenotypic heterogeneity in benign prostatic hyperplasia. Differentiation. 2017; 96:49–61.
Article5. Vignozzi L, Rastrelli G, Corona G, Gacci M, Forti G, Maggi M. Benign prostatic hyperplasia: a new metabolic disease? J Endocrinol Invest. 2014; 37:313–322.
Article6. Steers WD. 5alpha-reductase activity in the prostate. Urology. 2001; 58:17–24.7. Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011; 82:184–199.
Article8. Andriole G, Bruchovsky N, Chung LW, Matsumoto AM, Rittmaster R, Roehrborn C, Russell D, Tindall D. Dihydrotestosterone and the prostate: the scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol. 2004; 172:1399–1403.
Article9. Azzouni F, Mohler J. Role of 5α-reductase inhibitors in benign prostatic diseases. Prostate Cancer Prostatic Dis. 2012; 15:222–230.
Article10. Gharaee-Kermani M, Macoska JA. Promising molecular targets and biomarkers for male BPH and LUTS. Curr Urol Rep. 2013; 14:628–637.
Article11. Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011; 8:29–41.
Article12. Thorner DA, Weiss JP. Benign prostatic hyperplasia: symptoms, symptom scores, and outcome measures. Urol Clin North Am. 2009; 36:417–429.
Article13. Smith RD, Patel A. Transurethral resection of the prostate revisited and updated. Curr Opin Urol. 2011; 21:36–41.
Article14. Traish AM, Melcangi RC, Bortolato M, Garcia-Segura LM, Zitzmann M. Adverse effects of 5α-reductase inhibitors: what do we know, don't know, and need to know? Rev Endocr Metab Disord. 2015; 16:177–198.
Article15. Lepor H. Alpha-blockers for the treatment of benign prostatic hyperplasia. Urol Clin North Am. 2016; 43:311–323.
Article16. Chang JS, Chiang LC, Hsu FF, Lin CC. Chemoprevention against hepatocellular carcinoma of Cornus officinalis in vitro. Am J Chin Med. 2004; 32:717–725.
Article17. Kang DG, Moon MK, Lee AS, Kwon TO, Kim JS, Lee HS. Cornuside suppresses cytokine-induced proinflammatory and adhesion molecules in the human umbilical vein endothelial cells. Biol Pharm Bull. 2007; 30:1796–1799.
Article18. Jiang WL, Chen XG, Zhu HB, Hou J, Tian JW. Cornuside attenuates apoptosis and ameliorates mitochondrial energy metabolism in rat cortical neurons. Pharmacology. 2009; 84:162–170.
Article19. Jeong EJ, Kim TB, Yang H, Kang SY, Kim SY, Sung SH, Kim YC. Neuroprotective iridoid glycosides from Cornus officinalis fruits against glutamate-induced toxicity in HT22 hippocampal cells. Phytomedicine. 2012; 19:317–321.
Article20. Telang NT, Li G, Sepkovic DW, Bradlow HL, Wong GY. Antiproliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor-positive clinical breast cancer. Mol Med Rep. 2012; 5:22–28.21. Zhang QC, Zhao Y, Bian HM. Antiplatelet activity of a novel formula composed of malic acid, succinic acid and citric acid from Cornus officinalis fruit. Phytother Res. 2013; 27:1894–1896.
Article22. Sun X, Kong L, Zhou L. Protective effect of Fructus corni polysaccharide on hippocampal tissues and its relevant mechanism in epileptic rats induced by lithium chloride-pilocarpine. Exp Ther Med. 2018; 16:445–451.
Article23. Hwang KA, Hwang YJ, Song J. Antioxidant activities and oxidative stress inhibitory effects of ethanol extracts from Cornus officinalis on raw 264.7 cells. BMC Complement Altern Med. 2016; 16:196.
Article24. Park JY, Han AR, Kil YS, Kang U, Kim SH, Nam SJ, Seo EK. A new secoiridoid glycoside from the fruits of Cornus officinalis (Cornaceae). Nat Prod Res. 2016; 30:1504–1510.
Article25. Yoon JH, Youn K, Ho CT, Karwe MV, Jeong WS, Jun M. p-Coumaric acid and ursolic acid from Corni fructus attenuated β-amyloid(25-35)-induced toxicity through regulation of the NF-κB signaling pathway in PC12 cells. J Agric Food Chem. 2014; 62:4911–4916.
Article26. Ma W, Wang KJ, Cheng CS, Yan GQ, Lu WL, Ge JF, Cheng YX, Li N. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J Ethnopharmacol. 2014; 153:840–845.
Article27. Minciullo PL, Inferrera A, Navarra M, Calapai G, Magno C, Gangemi S. Oxidative stress in benign prostatic hyperplasia: a systematic review. Urol Int. 2015; 94:249–254.
Article28. Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol. 2013; 23:5–10.
Article29. Keam SJ, Scott LJ. Dutasteride: a review of its use in the management of prostate disorders. Drugs. 2008; 68:463–485.30. Traish AM. 5α-reductases in human physiology: an unfolding story. Endocr Pract. 2012; 18:965–975.
Article31. Pihlajamaa P, Sahu B, Jänne OA. Determinants of receptor- and tissue-specific actions in androgen signaling. Endocr Rev. 2015; 36:357–384.
Article32. Robins DM. Androgen receptor and molecular mechanisms of male-specific gene expression. Novartis Found Symp. 2005; 268:42–52.
Article33. Prins GS. Molecular biology of the androgen receptor. Mayo Clin Proc. 2000; 75:Suppl. S32–S35.
Article34. Watanabe M, Yamada Y, Kato H, Imai H, Nakano H, Araki T, Shiraishi T. Malignant phyllodes tumor of the prostate: retrospective review of specimens obtained by sequential transurethral resection. Pathol Int. 2002; 52:777–783.
Article35. Cunha GR, Wang YZ, Hayward SW, Risbridger GP. Estrogenic effects on prostatic differentiation and carcinogenesis. Reprod Fertil Dev. 2001; 13:285–296.
Article36. Huang J, Zhang Y, Dong L, Gao Q, Yin L, Quan H, Chen R, Fu X, Lin D. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J Ethnopharmacol. 2018; 213:280–301.
Article37. Park CH, Noh JS, Park JC, Yokozawa T. Beneficial effect of 7-O-galloyl-D-sedoheptulose, a polyphenol isolated from Corni fructus, against diabetes-induced alterations in kidney and adipose tissue of type 2 diabetic db/db mice. Evid Based Complement Alternat Med. 2013; 2013:736856.38. Vareed SK, Schutzki RE, Nair MG. Lipid peroxidation, cyclooxygenase enzyme and tumor cell proliferation inhibitory compounds in Cornus kousa fruits. Phytomedicine. 2007; 14:706–709.
Article39. Wang W, Sun F, An Y, Ai H, Zhang L, Huang W, Li L. Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur J Pharmacol. 2009; 613:19–23.
Article40. Xu H, Shen J, Liu H, Shi Y, Li L, Wei M. Morroniside and loganin extracted from Cornus officinalis have protective effects on rat mesangial cell proliferation exposed to advanced glycation end products by preventing oxidative stress. Can J Physiol Pharmacol. 2006; 84:1267–1273.
Article41. Xu YD, Cui C, Sun MF, Zhu YL, Chu M, Shi YW, Lin SL, Yang XS, Shen YQ. Neuroprotective effects of loganin on MPTP-induced Parkinson's disease mice: neurochemistry, glial reaction and autophagy studies. J Cell Biochem. 2017; 118:3495–3510.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mixture of Corni Fructus and Schisandrae Fructus improves testosterone-induced benign prostatic hyperplasia through regulating 5α-reductase 2 and androgen receptor
- Albizzia julibrissin Suppresses Testosterone-induced Benign Prostatic Hyperplasia by Regulating 5α-Reductase Type 2 – Androgen Receptor Pathway
- 5alpha-Reductase
- Effect of Progesterone on COX-2 Expression and Proliferation of Prostate Stromal Cell
- The Antihyperplastic Effect of Oral Catechin Ingestion in a Rat Model of Benign Prostatic Hyperplasia