Nutr Res Pract.
2023 Feb;17(1):32-47. 10.4162/nrp.2023.17.1.32.
Mixture of Corni Fructus and Schisandrae Fructus improves testosterone-induced benign prostatic hyperplasia through regulating 5α-reductase 2 and androgen receptor
- Affiliations
-
- 1Korea Nanobiotechnology Center, Pusan National University, Busan 46241, Korea
- 2Department of Biochemistry, Dong-eui University College of Korean Medicine, Busan 47227, Korea
- 3Anti-Aging Research Center, Dong-eui University, Busan 47340, Korea
- 4Hamsoapharm Central Research, Jinan 55442, Korea
- 5R&D Center, Naturetech Co. Ltd., Jincheon 27858, Korea
- 6BIO Center, Chungbuk Technopark, Ochang 28115, Korea
- 7Department of Marine Life Science, Jeju National University, Jeju 63243, Korea
- KMID: 2539451
- DOI: http://doi.org/10.4162/nrp.2023.17.1.32
Abstract
- BACKGROUND/OBJECTIVES
Benign prostatic hyperplasia (BPH) characterized by an enlarged prostate gland is common in elderly men. Corni Fructus (CF) and Schisandrae Fructus (SF) are known to have various pharmacological effects, including antioxidant and anti-inflammatory activities. In this study, we evaluated the inhibitory efficacy of CF, SF, and their mixture (MIX) on the development of BPH using an in vivo model of testosteroneinduced BPH.
MATERIALS/METHODS
Six-week-old male Sprague-Dawley rats were randomly divided into seven groups. To induce BPH, testosterone propionate (TP) was injected to rats except for those in the control group. Finasteride, saw palmetto (SP), CF, SF, and MIX were orally administered along with TP injection. At the end of treatment, histological changes in the prostate and the level of various biomarkers related to BPH were evaluated.
RESULTS
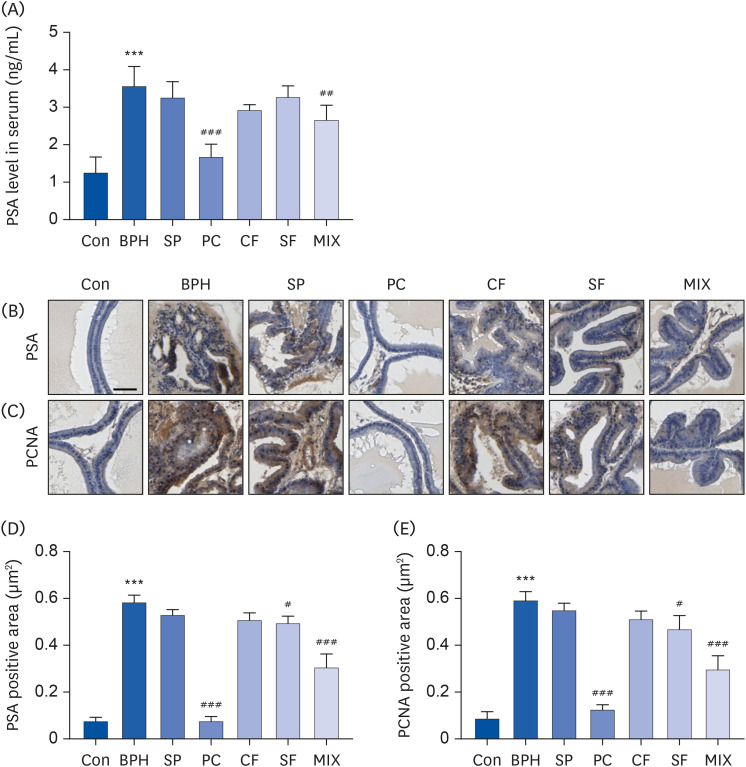
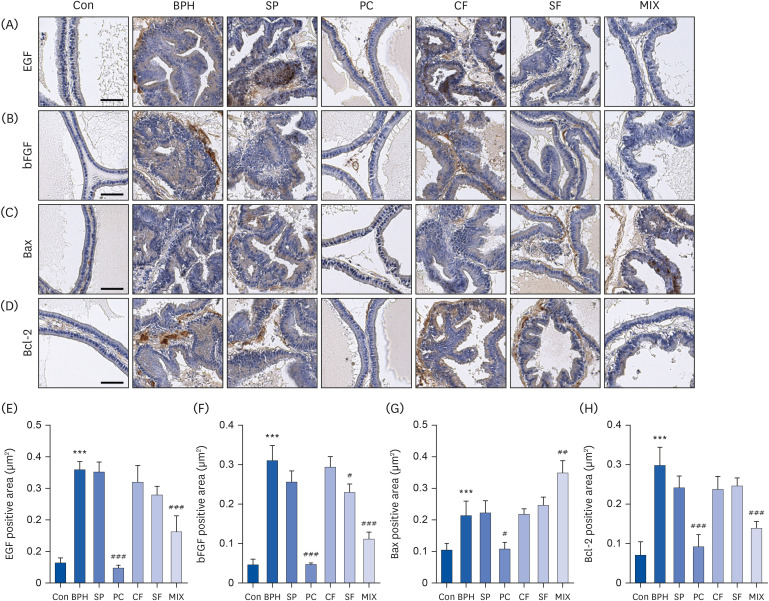
Our results showed that BPH induced by TP led to prostate weight and histological changes. Treatment with MIX effectively improved TP-induced BPH by reducing prostate index, lumen area, epithelial thickness, and expression of BPH biomarkers such as 5α-reductase type 2, prostate-specific antigen, androgen receptor, and proliferating cell nuclear antigen compared to treatment with CF or SF alone. Moreover, MIX further reduced levels of elevated serum testosterone, dihydrotestosterone, and prostate-specific antigen in BPH compared to the SP, a positive control. BPH was also improved more by MIX than by CF or SF alone.
CONCLUSIONS
Based on the results, MIX is a potential natural therapeutic candidate for BPH by regulating 5α-reductase and AR signaling pathway.
Keyword
Figure
Cited by 1 articles
-
Asparagi radix alleviates testosterone-induced benign prostatic hyperplasia by inhibiting 5α-reductase activity and androgen receptor signaling pathway
Hyun Hwangbo, Hee-Jae Cha, Min Yeong Kim, Seon Yeong Ji, Da Hye Kim, Jeong Sook Noh, Tae Hee Kim, Heui-Soo Kim, Sung-Kwon Moon, Gi-Young Kim, Yung Hyun Choi
Nutr Res Pract. 2024;18(6):793-805. doi: 10.4162/nrp.2024.18.6.793.
Reference
-
1. Sharma M, Chadha R, Dhingra N. Phytotherapeutic agents for benign prostatic hyperplasia: an overview. Mini Rev Med Chem. 2017; 17:1346–1363. PMID: 27337973.2. Vickman RE, Franco OE, Moline DC, Vander Griend DJ, Thumbikat P, Hayward SW. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: a review. Asian J Urol. 2020; 7:191–202. PMID: 32742923.3. Vasanwala FF, Wong MY, Ho HS, Foo KT. Benign prostatic hyperplasia and male lower urinary symptoms: a guide for family physicians. Asian J Urol. 2017; 4:181–184. PMID: 29264228.4. Mobley D, Feibus A, Baum N. Benign prostatic hyperplasia and urinary symptoms: evaluation and treatment. Postgrad Med. 2015; 127:301–307. PMID: 25823641.5. Madersbacher S, Sampson N, Culig Z. Pathophysiology of benign prostatic hyperplasia and benign prostatic enlargement: a mini-review. Gerontology. 2019; 65:458–464. PMID: 30943489.6. Zhu YS, Imperato-McGinley JL. 5alpha-reductase isozymes and androgen actions in the prostate. Ann N Y Acad Sci. 2009; 1155:43–56. PMID: 19250191.7. Cózar JM, Hernández C, Miñana B, Morote J, Alvarez-Cubero MJ. The role of prostate-specific antigen in light of new scientific evidence: an update in 2020. Actas Urol Esp (Engl Ed). 2021; 45:21–29. PMID: 33408046.8. Kizilay F, Kalemci MS, Şimşir A, Turna B, Nazli O, Berdeli A. The place of androgen receptor gene mutation analysis in the molecular diagnosis of prostate cancer and genotype-phenotype relationship. Turk J Med Sci. 2014; 44:261–266. PMID: 25536734.9. La Vignera S, Aversa A, Cannarella R, Condorelli RA, Duca Y, Russo GI, Calogero AE. Pharmacological treatment of lower urinary tract symptoms in benign prostatic hyperplasia: consequences on sexual function and possible endocrine effects. Expert Opin Pharmacother. 2021; 22:179–189. PMID: 32902360.10. De Nunzio C, Presicce F, Tubaro A. Combination therapies for improved management of lower urinary tract symptoms/benign prostatic hyperplasia. Drugs Today (Barc). 2016; 52:501–517. PMID: 27883117.11. Gupta S, Gupta G, Sharma VL. Evolving novel chemical entities for management of benign prostatic hyperplasia. Mini Rev Med Chem. 2017; 17:593–602. PMID: 27484626.12. Garg G, Singh D, Saraf S, Saraf S. Management of benign prostate hyperplasia: an overview of alpha-adrenergic antagonist. Biol Pharm Bull. 2006; 29:1554–1558. PMID: 16880603.13. Azzouni F, Mohler J. Role of 5α-reductase inhibitors in benign prostatic diseases. Prostate Cancer Prostatic Dis. 2012; 15:222–230. PMID: 22333687.14. Traish AM, Mulgaonkar A, Giordano N. The dark side of 5α-reductase inhibitors’ therapy: sexual dysfunction, high Gleason grade prostate cancer and depression. Korean J Urol. 2014; 55:367–379. PMID: 24955220.15. Gao X, Liu Y, An Z, Ni J. Active components and pharmacological effects of Cornus officinalis: literature review. Front Pharmacol. 2021; 12:633447. PMID: 33912050.16. Dong Y, Feng ZL, Chen HB, Wang FS, Lu JH. Corni Fructus: a review of chemical constituents and pharmacological activities. Chin Med. 2018; 13:34. PMID: 29983732.17. Kopustinskiene DM, Bernatoniene J. Antioxidant effects of Schisandra chinensis fruits and their active constituents. Antioxidants. 2021; 10:620. PMID: 33919588.18. Rybnikář M, Šmejkal K, Žemlička M. Schisandra chinensis and its phytotherapeutical applications. Ceska Slov Farm. 2019; 68:95–118. PMID: 31431019.19. Huang J, Zhang Y, Dong L, Gao Q, Yin L, Quan H, Chen R, Fu X, Lin D. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J Ethnopharmacol. 2018; 213:280–301. PMID: 29155174.20. Zhou Y, Men L, Sun Y, Wei M, Fan X. Pharmacodynamic effects and molecular mechanisms of lignans from Schisandra chinensis Turcz. (Baill.), a current review. Eur J Pharmacol. 2021; 892:173796. PMID: 33345853.21. Mitsunari K, Miyata Y, Matsuo T, Mukae Y, Otsubo A, Harada J, Kondo T, Matsuda T, Ohba K, Sakai H. Pharmacological effects and potential clinical usefulness of polyphenols in benign prostatic hyperplasia. Molecules. 2021; 26:450. PMID: 33467066.22. Castelli T, Russo GI, Reale G, Privitera S, Chisari M, Fragalà E, Favilla V, Cimino S, Morgia G. Metabolic syndrome and prostatic disease: potentially role of polyphenols in preventive strategies. A review. Int Braz J Urol. 2016; 42:422–430. PMID: 27286103.23. Minciullo PL, Inferrera A, Navarra M, Calapai G, Magno C, Gangemi S. Oxidative stress in benign prostatic hyperplasia: a systematic review. Urol Int. 2015; 94:249–254. PMID: 25503259.24. Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol. 2013; 23:5–10. PMID: 23159991.25. Hwangbo H, Kwon DH, Choi EO, Kim MY, Ahn KI, Ji SY, Kim JS, Kim KI, Park NJ, Kim BH, et al. Corni Fructus attenuates testosterone-induced benign prostatic hyperplasia by suppressing 5α-reductase and androgen receptor expression in rats. Nutr Res Pract. 2018; 12:378–386. PMID: 30323905.26. Wei F, He X, Xu K, Wang S. Stepwise frontal analysis coupled with cell membrane chromatography for affinity screening and characterization analysis of bioactive constituent from the mature fruits of schisandra chinensis . J Chromatogr B Analyt Technol Biomed Life Sci. 2020; 1161:122443.27. Bandiera S, Pulcinelli RR, Huf F, Almeida FB, Halmenschlager G, Bitencourt PE, Dallegrave E, C Fernandes M, Gomez R, Nin MS. Hepatic and renal damage by alcohol and cigarette smoking in rats. Toxicol Res. 2020; 37:209–219. PMID: 33868978.28. Bhat A, Blachman-Braun R, Herrmann TR, Shah HN. Are all procedures for benign prostatic hyperplasia created equal? A systematic review on post-procedural PSA dynamics and its correlation with relief of bladder outlet obstruction. World J Urol. 2022; 40:889–905. PMID: 34212237.29. Gravina GL, Mancini A, Ranieri G, Di Pasquale B, Marampon F, Di Clemente L, Ricevuto E, Festuccia C. Phenotypic characterization of human prostatic stromal cells in primary cultures derived from human tissue samples. Int J Oncol. 2013; 42:2116–2122. PMID: 23589051.30. Zhou Y, Xiao XQ, Chen LF, Yang R, Shi JD, Du XL, Klocker H, Park I, Lee C, Zhang J. Proliferation and phenotypic changes of stromal cells in response to varying estrogen/androgen levels in castrated rats. Asian J Androl. 2009; 11:451–459. PMID: 19483715.31. Rodriguez-Nieves JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol. 2013; 10:546–550. PMID: 23857178.32. Song KH, Seo CS, Yang WK, Gu HO, Kim KJ, Kim SH. Extracts of Phyllostachys pubescens leaves represses human steroid 5-α reductase type 2 promoter activity in BHP-1 cells and ameliorates testosterone-induced benign prostatic hyperplasia in rat model. Nutrients. 2021; 13:884. PMID: 33803357.33. Kim DG, Kwon HJ, Lim JH, Kim JH, Lee KP. Quisqualis indica extract ameliorates low urinary tract symptoms in testosterone propionate-induced benign prostatic hyperplasia rats. Lab Anim Res. 2020; 36:26. PMID: 32793460.34. Kim HJ, Jin BR, An HJ. Psoralea corylifolia L. extract ameliorates benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. J Ethnopharmacol. 2021; 273:113844. PMID: 33485982.35. Hong GL, Park SR, Jung DY, Karunasagara S, Lee KP, Koh EJ, Cho K, Park SS, Jung JY. The therapeutic effects of Stauntonia hexaphylla in benign prostate hyperplasia are mediated by the regulation of androgen receptors and 5α-reductase type 2. J Ethnopharmacol. 2020; 250:112446. PMID: 31812646.36. Choi YJ, Kim EK, Fan M, Tang Y, Hwang YJ, Sung SH. Effect of Paecilomyces tenuipes extract on testosterone-induced benign prostatic hyperplasia in Sprague-Dawley rats. Int J Environ Res Public Health. 2019; 16:3764. PMID: 31591335.37. Lee G, Shin J, Choi H, Jo A, Pan S, Bae D, Lee Y, Choi C. Cynanchum wilfordii ameliorates testosterone-induced benign prostatic hyperplasia by regulating 5α-reductase and androgen receptor activities in a rat model. Nutrients. 2017; 9:1070. PMID: 28953224.38. Karunasagara S, Hong GL, Jung DY, Kim KH, Cho K, Jung JY. Protective effects of combination of Stauntonia hexaphylla and Cornus officinalis on testosterone-induced benign prostatic hyperplasia through inhibition of 5α- reductase type 2 and induced cell apoptosis. PLoS One. 2020; 15:e0236879. PMID: 32790676.39. Uroko RI, Chukwu CN, Egba SI, Adamude FA, Ajuzie JC. Combined ethanol extract of Funtumia africana and Abutilon mauritianium leaves improves the lipid profile and kidney function indices of benign prostatic hyperplasia in rats. Acta Sci Pol Technol Aliment. 2020; 19:395–404. PMID: 33179480.40. Cai H, Cao G, Cai B. Rapid simultaneous identification and determination of the multiple compounds in crude Fructus Corni and its processed products by HPLC-MS/MS with multiple reaction monitoring mode. Pharm Biol. 2013; 51:273–278. PMID: 23134086.41. Liu H, Lai H, Jia X, Liu J, Zhang Z, Qi Y, Zhang J, Song J, Wu C, Zhang B, et al. Comprehensive chemical analysis of Schisandra chinensis by HPLC-DAD-MS combined with chemometrics. Phytomedicine. 2013; 20:1135–1143. PMID: 23880331.42. Kim DU, Kim DG, Choi JW, Shin JY, Kweon B, Zhou Z, Lee HS, Song HJ, Bae GS, Park SJ. Loganin attenuates the severity of acute kidney injury induced by cisplatin through the inhibition of ERK activation in mice. Int J Mol Sci. 2021; 22:1421. PMID: 33572597.43. Cheng YC, Chiu YM, Dai ZK, Wu BN. Loganin ameliorates painful diabetic neuropathy by modulating oxidative stress, inflammation and insulin sensitivity in streptozotocin-nicotinamide-induced diabetic rats. Cells. 2021; 10:2688. PMID: 34685668.44. Nasser MI, Han T, Adlat S, Tian Y, Jiang N. Inhibitory effects of Schisandrin B on human prostate cancer cells. Oncol Rep. 2019; 41:677–685. PMID: 30320364.45. Mahapokai W, Van Sluijs FJ, Schalken JA. Models for studying benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2000; 3:28–33. PMID: 12497158.46. Zhang J, Zhang M, Tang J, Yin G, Long Z, He L, Zhou C, Luo L, Qi L, Wang L. Animal models of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2021; 24:49–57. PMID: 32873917.47. Soni KK, Shin YS, Choi BR, Karna KK, Kim HK, Lee SW, Kim CY, Park JK. Protective effect of DA-9401 in finasteride-induced apoptosis in rat testis: inositol requiring kinase 1 and c-Jun N-terminal kinase pathway. Drug Des Devel Ther. 2017; 11:2969–2979.48. Messner EA, Steele TM, Tsamouri MM, Hejazi N, Gao AC, Mudryj M, Ghosh PM. The androgen receptor in prostate cancer: effect of structure, ligands and spliced variants on therapy. Biomedicines. 2020; 8:422. PMID: 33076388.49. Chughtai B, Forde JC, Thomas DD, Laor L, Hossack T, Woo HH, Te AE, Kaplan SA. Benign prostatic hyperplasia. Nat Rev Dis Primers. 2016; 2:16031. PMID: 27147135.50. Soulitzis N, Karyotis I, Delakas D, Spandidos DA. Expression analysis of peptide growth factors VEGF, FGF2, TGFB1, EGF and IGF1 in prostate cancer and benign prostatic hyperplasia. Int J Oncol. 2006; 29:305–314. PMID: 16820871.51. Minutoli L, Rinaldi M, Marini H, Irrera N, Crea G, Lorenzini C, Puzzolo D, Valenti A, Pisani A, Adamo EB, et al. Apoptotic pathways linked to endocrine system as potential therapeutic targets for benign prostatic hyperplasia. Int J Mol Sci. 2016; 17:1311. PMID: 27529214.52. Jin BR, Cheon SY, Kim HJ, Kim MS, Lee KH, An HJ. Anti-proliferative effects of standardized Cornus officinalis on benign prostatic epithelial cells via the PCNA/E2F1-dependent cell cycle pathway. Int J Mol Sci. 2020; 21:9567. PMID: 33334082.53. Choo SH, Sung HH, Chae MR, Kang SJ, Han DH, Park JK, So I, Lee SW. Effects of Schisandra chinensis extract on the relaxation of isolated human prostate tissue and smooth muscle cell. J Ethnopharmacol. 2014; 156:271–276. PMID: 25178950.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corni Fructus attenuates testosterone-induced benign prostatic hyperplasia by suppressing 5α-reductase and androgen receptor expression in rats
- 5alpha-Reductase
- Antidiabetic Effects of Corni Fructus Extract in Streptozotocin-Induced Diabetic Rats
- Corni Fructus-Induced Acute Interstitial Nephritis
- Asparagi radix alleviates testosterone-induced benign prostatic hyperplasia by inhibiting 5α-reductase activity and androgen receptor signaling pathway