Yonsei Med J.
2012 Jul;53(4):691-700. 10.3349/ymj.2012.53.4.691.
Antidiabetic Effects of Corni Fructus Extract in Streptozotocin-Induced Diabetic Rats
- Affiliations
-
- 1Department of Biological Engineering, College of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao, China. dwgao@ysu.edu.cn
- 2Department of Surgery, Beijing Ditan Hospital, Beijing, China.
- KMID: 1716865
- DOI: http://doi.org/10.3349/ymj.2012.53.4.691
Abstract
- PURPOSE
Diabetes is the leading cause of end-stage renal failure. The present study was undertaken to characterize the effects of Corni Fructus on diabetic nephropathy in streptozotocin-induced diabetic rats and their mechanisms.
MATERIALS AND METHODS
Streptozotocin-diabetic rats were orally administrated with Corni Fructus at a dose of 100, 200 or 400 mg/kg body mass for 40 days.
RESULTS
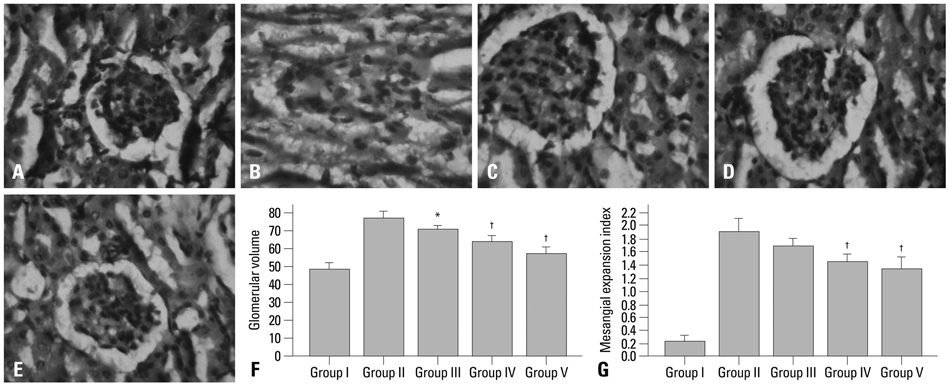
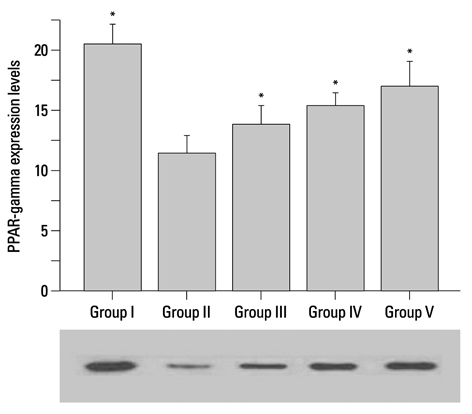
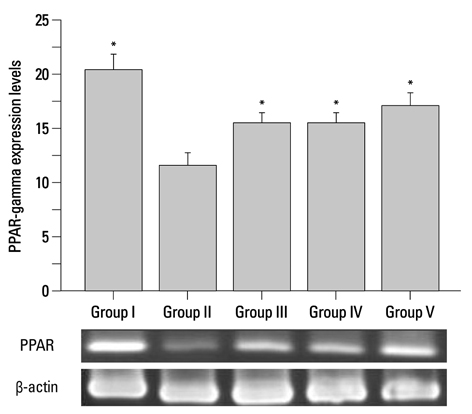
Corni Fructus-treated diabetic rats showed significant decreases of blood glucose, urinary protein levels and water consumption. Corni Fructus also reduced serum total cholesterol, total triglyceride and low-density lipoprotein cholesterol levels, and showed a tendency of enhancing high-density lipoprotein cholesterol level. Levels of serum albumin and creatinine in diabetic rats were also significantly reduced by Corni Fructus administration at a dose of 200 and 400 mg/kg body mass compared with non-treated diabetic rats. Corni Fructus increased catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidose (GSH-px) activities in the kidneys of diabetic rats. Furthermore, Corni Fructus treatment enhanced renal peroxisome proliferator-activated receptor-gamma (PPARgamma) expression in diabetic rats.
CONCLUSION
These results demonstrated that Corni Fructus may have the potential to protect the animals from diabetic nephropathy by amelioration of oxidative stress and stimulation of PPARgamma expression.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Body Weight/drug effects
Catalase/metabolism
Cornus/*chemistry
Diabetes Mellitus, Experimental/*drug therapy
Glucose Tolerance Test
Glutathione/metabolism
Hypoglycemic Agents/*therapeutic use
Male
Malondialdehyde/metabolism
Plant Extracts/*therapeutic use
Rats
Rats, Wistar
Reverse Transcriptase Polymerase Chain Reaction
Superoxide Dismutase/metabolism
Figure
Reference
-
1. Cooper ME, Allen TJ, O'Brien RC, Macmillan PA, Clarke B, Jerums G, et al. Effects of genetic hypertension on diabetic nephropathy in the rat--functional and structural characteristics. J Hypertens. 1988. 6:1009–1016.
Article2. Prabhakar S, Starnes J, Shi S, Lonis B, Tran R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J Am Soc Nephrol. 2007. 18:2945–2952.
Article3. Ceriello A, Bortolotti N, Falleti E, Taboga C, Tonutti L, Crescentini A, et al. Total radical-trapping antioxidant parameter in NIDDM patients. Diabetes Care. 1997. 20:194–197.
Article4. Yang HC, Ma LJ, Ma J, Fogo AB. Peroxisome proliferator-activated receptor-gamma agonist is protective in podocyte injury-associated sclerosis. Kidney Int. 2006. 69:1756–1764.
Article5. Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005. 437:759–763.
Article6. Rubenstrunk A, Hanf R, Hum DW, Fruchart JC, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta. 2007. 1771:1065–1081.
Article7. Kuroe A, Taniuguchi A, Fukushima M, Nakai Y, Ohgushi M, Ohya M, et al. Early and late onset side effects of short-acting insulin analogue in seven Japanese diabetic patients. Diabetes Res Clin Pract. 2007. 77:412–413.
Article8. Shoji Y, Nakashima H. Glucose-lowering effect of powder formulation of African black tea extract in KK-A(y)/TaJcl diabetic mouse. Arch Pharm Res. 2006. 29:786–794.
Article9. Kochhar A, Nagi M. Effect of supplementation of traditional medicinal plants on blood glucose in non-insulin-dependent diabetics: a pilot study. J Med Food. 2005. 8:545–549.
Article10. Qian DS, Zhu YF, Zhu Q. [Effect of alcohol extract of Cornus officinalis Sieb. et Zucc on GLUT4 expression in skeletal muscle in type 2 (non-insulin-dependent) diabetic mellitus rats]. Zhongguo Zhong Yao Za Zhi. 2001. 26:859–862.11. Wu VC, Qiu X, Peggy Hsieh YH. Evaluation of Escherichia coli O157:H7 in apple juice with Cornus fruit (Cornus officinalis Sieb. et Zucc.) extract by conventional media and thin agar layer method. Food Microbiol. 2008. 25:190–195.
Article12. Peng Q, Wei Z, Lau BH. Fructus corni attenuates oxidative stress in macrophages and endothelial cells. Am J Chin Med. 1998. 26:291–300.
Article13. Gao D, Li N, Li Q, Li J, Han Z, Fan Y, et al. Study of the extraction, purification and antidiabetic potential of ursolic acid from Cornus officinalis Sieb. et Zucc. Therapy. 2008. 5:697–705.
Article14. Gao D, Li Q, Li Y, Liu Z, Liu Z, Fan Y, et al. Antidiabetic potential of oleanolic acid from Ligustrum lucidum Ait. Can J Physiol Pharmacol. 2007. 85:1076–1083.15. Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969. 22:158–161.
Article16. Asami-Miyagishi R, Iseki S, Usui M, Uchida K, Kubo H, Morita I. Expression and function of PPARgamma in rat placental development. Biochem Biophys Res Commun. 2004. 315:497–501.17. van Bamme B, Koudstaal J. Measuring glomerular diameters in tissue sections. Virchows Arch A Pathol Anat Histol. 1976. 369:283–291.
Article18. Liu IM, Tzeng TF, Liou SS, Chang CJ. The amelioration of streptozotocin diabetes-induced renal damage by Wu-Ling-San (Hoelen Five Herb Formula), a traditional Chinese prescription. J Ethnopharmacol. 2009. 124:211–218.
Article19. Shapiro K, Gong WC. Natural products used for diabetes. J Am Pharm Assoc (Wash). 2002. 42:217–226.
Article20. Ajjan RA, Grant PJ. Cardiovascular disease prevention in patients with type 2 diabetes: the role of oral anti-diabetic agents. Diab Vasc Dis Res. 2006. 3:147–158.
Article21. Ozsoy-Sacan O, Karabulut-Bulan O, Bolkent S, Yanardag R, Ozgey Y. Effects of chard (Beta vulgaris L. var cicla) on the liver of the diabetic rats: a morphological and biochemical study. Biosci Biotechnol Biochem. 2004. 68:1640–1648.
Article22. Technical report series 646. WHO Expert Committee on Diabetes Mellitus: Second Report. 1980. Geneva, Switzerland: World Health Organization;18.23. Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P. Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001. 345:870–878.
Article24. Ahn CW, Song YD, Kim JH, Lim SK, Choi KH, Kim KR, et al. The validity of random urine specimen albumin measurement as a screening test for diabetic nephropathy. Yonsei Med J. 1999. 40:40–45.
Article25. Falk RJ, Scheinman JI, Mauer SM, Michael AF. Polyantigenic expansion of basement membrane constituents in diabetic nephropathy. Diabetes. 1983. 32:Suppl 2. 34–39.
Article26. Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000. 49:157–162.
Article27. Chen V, Ianuzzo CD. Dosage effect of streptozotocin on rat tissue enzyme activities and glycogen concentration. Can J Physiol Pharmacol. 1982. 60:1251–1256.
Article28. Rabinovitch A, Suarez WL, Thomas PD, Strynadka K, Simpson I. Cytotoxic effects of cytokines on rat islets: evidence for involvement of free radicals and lipid peroxidation. Diabetologia. 1992. 35:409–413.
Article29. Reddy SV, Tiwari AK, Kumar US, Rao RJ, Rao JM. Free radical scavenging, enzyme inhibitory constituents from antidiabetic Ayurvedic medicinal plant Hydnocarpus wightiana Blume. Phytother Res. 2005. 19:277–281.
Article30. Hunt JV, Smith CC, Wolff SP. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990. 39:1420–1424.
Article31. Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab. 2000. 26:163–176.32. Zheng F, Fornoni A, Elliot SJ, Guan Y, Breyer MD, Striker LJ, et al. Upregulation of type I collagen by TGF-beta in mesangial cells is blocked by PPARgamma activation. Am J Physiol Renal Physiol. 2002. 282:F639–F648.33. Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002. 53:409–435.
Article34. Hamm JK, el Jack AK, Pilch PF, Farmer SR. Role of PPAR gamma in regulating adipocyte differentiation and insulin-responsive glucose uptake. Ann N Y Acad Sci. 1999. 892:134–145.
Article35. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994. 79:1147–1156.
Article36. Iwata M, Haruta T, Usui I, Takata Y, Takano A, Uno T, et al. Pioglitazone ameliorates tumor necrosis factor-alpha-induced insulin resistance by a mechanism independent of adipogenic activity of peroxisome proliferator--activated receptor-gamma. Diabetes. 2001. 50:1083–1092.
Article37. Li D, Wang X, Ren W, Ren J, Lan X, Wang F, et al. High expression of liver histone deacetylase 3 contributes to high-fat-diet-induced metabolic syndrome by suppressing the PPAR-γ and LXR-α-pathways in E3 rats. Mol Cell Endocrinol. 2011. 344:69–80.
Article38. Zhang H, Saha J, Byun J, Schin M, Lorenz M, Kennedy RT, et al. Rosiglitazone reduces renal and plasma markers of oxidative injury and reverses urinary metabolite abnormalities in the amelioration of diabetic nephropathy. Am J Physiol Renal Physiol. 2008. 295:F1071–F1081.
Article39. Rose M, Balakumar P, Singh M. Ameliorative effect of combination of fenofibrate and rosiglitazone in pressure overload-induced cardiac hypertrophy in rats. Pharmacology. 2007. 80:177–184.
Article40. Lee KS, Kim SR, Park SJ, Park HS, Min KH, Jin SM, et al. Peroxisome proliferator activated receptor-gamma modulates reactive oxygen species generation and activation of nuclear factor-kappaB and hypoxia-inducible factor 1alpha in allergic airway disease of mice. J Allergy Clin Immunol. 2006. 118:120–127.
Article41. Buckingham RE, Al-Barazanji KA, Toseland CD, Slaughter M, Connor SC, West A, et al. Peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, protects against nephropathy and pancreatic islet abnormalities in Zucker fatty rats. Diabetes. 1998. 47:1326–1334.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The antidiabetic effects of an herbal formula composed of Alnus hirsuta, Rosa davurica, Acanthopanax senticosus and Panax schinseng in the streptozotocin-induced diabetic rats
- Proliferation of Cultured Vascular Smooth Muscle Cells(VSMCs) Obtained from Aortas of Insulin Dependent Diabetic Rats
- Inhibitory Effect of Corni fructus on Compound 48/80-induced Mast Cell Activation and Vascular Permeability
- Corni Fructus-Induced Acute Interstitial Nephritis
- Inhibitory Effects of Corni Fructus Extract on Angiogenesis and Adipogenesis