Dement Neurocogn Disord.
2017 Sep;16(3):64-71. 10.12779/dnd.2017.16.3.64.
Candesartan Restores the Amyloid Beta-Inhibited Proliferation of Neural Stem Cells by Activating the Phosphatidylinositol 3-Kinase Pathway
- Affiliations
-
- 1Department of Neurology, Hanyang University Guri Hospital, Guri, Korea. ksh213@hanyang.ac.kr
- KMID: 2442818
- DOI: http://doi.org/10.12779/dnd.2017.16.3.64
Abstract
- BACKGROUND AND PURPOSE
Neurogenesis in the adult brain is important for memory and learning, and the alterations in neural stem cells (NSCs) may be an important aspect of Alzheimer's disease (AD) pathogenesis. The phosphatidylinositol 3-kinase (PI3K) pathway has been suggested to have an important role in neuronal cell survival and is highly involved in adult neurogenesis. Candesartan is an angiotensin II receptor antagonist used for the treatment of hypertension and several studies have reported that it also has some neuroprotective effects. We investigated whether candesartan could restore the amyloid-β(25-35) (Aβ₂₅₋₃₅) oligomer-inhibited proliferation of NSCs by focusing on the PI3K pathway.
METHODS
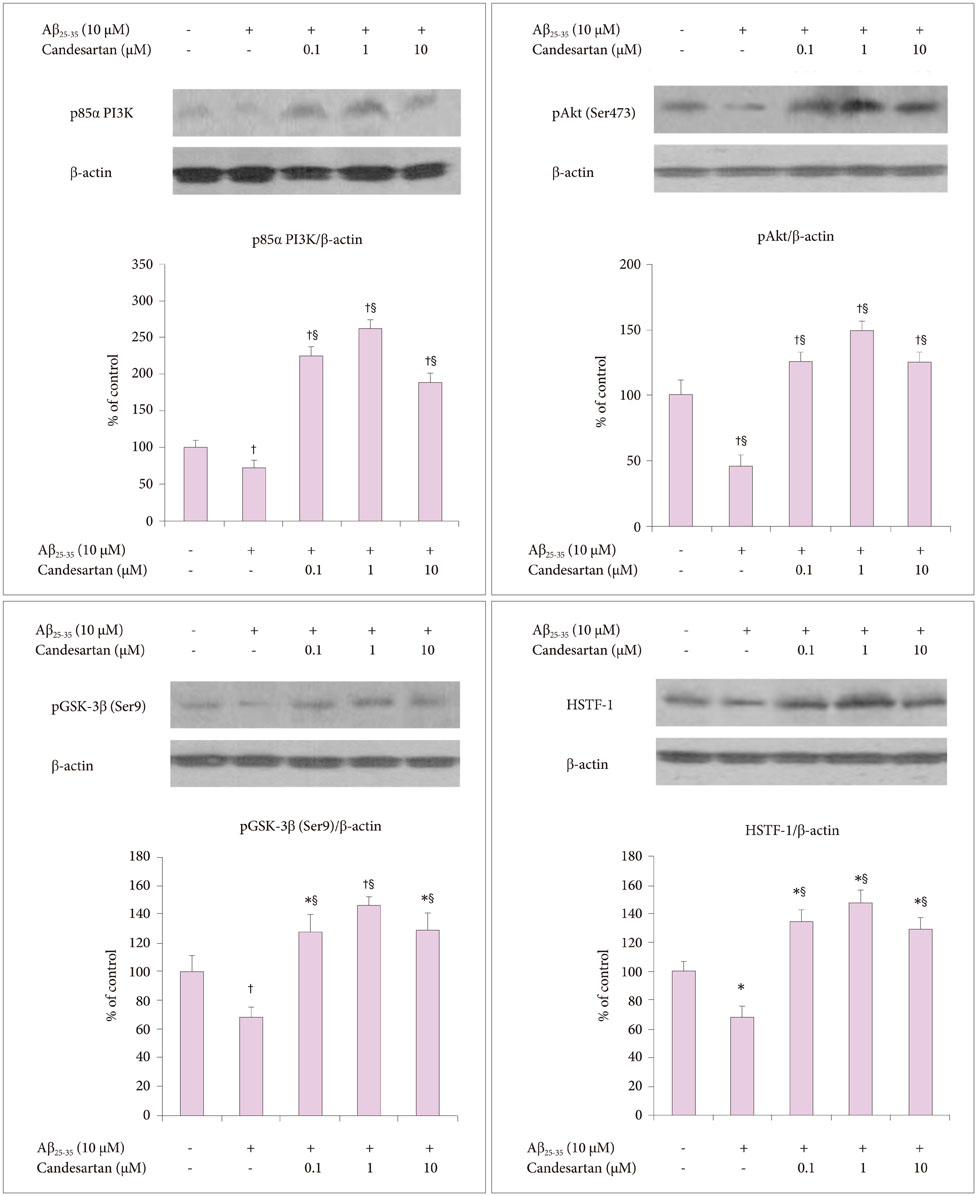
To evaluate the effects of candesartan on the Aβ₂₅₋₃₅ oligomer-inhibited proliferation of NSCs, the NSCs were treated with several concentrations of candesartan and/or Aβ₂₅₋₃₅ oligomers, and MTT assay and trypan blue staining were performed. To evaluate the effect of candesartan on the Aβ-inhibited proliferation of NSCs, we performed a bromodeoxyuridine (BrdU) labeling assay. The levels of p85α PI3K, phosphorylated Akt (pAkt) (Ser473), phosphorylated glycogen sinthase kinase-3β (pGSK-3β) (Ser9), and heat shock transcription factor-1 (HSTF-1) were analyzed by Western blotting.
RESULTS
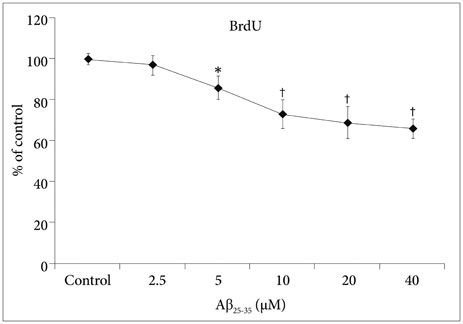
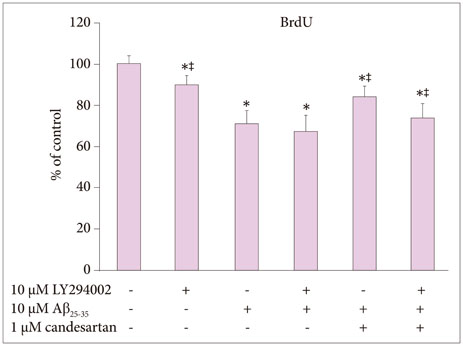
The BrdU assays demonstrated that NSC proliferation decreased with Aβ25-35 oligomer treatment; however, a combined treatment with candesartan restored it. Western blotting displayed that candesartan treatment increased the expression levels of p85α PI3K, pAkt (Ser473), pGSK-3β (Ser9), and HSTF. The NSCs were pretreated with a PI3K inhibitor, LY294002; the effects of candesartan on the proliferation of NSCs inhibited by Aβ₂₅₋₃₅ oligomers were almost completely blocked.
CONCLUSIONS
Together, these results suggest that candesartan restores the Aβ₂₅₋₃₅ oligomer-inhibited proliferation of NSCs by activating the PI3K pathway.
Keyword
MeSH Terms
-
Adult
Alzheimer Disease
Amyloid*
Blotting, Western
Brain
Bromodeoxyuridine
Cell Survival
Glycogen
Hot Temperature
Humans
Hypertension
Learning
Memory
Neural Stem Cells*
Neurogenesis
Neurons
Neuroprotective Agents
Phosphatidylinositol 3-Kinase*
Phosphatidylinositols*
Receptors, Angiotensin
Shock
Trypan Blue
Amyloid
Bromodeoxyuridine
Glycogen
Neuroprotective Agents
Phosphatidylinositol 3-Kinase
Phosphatidylinositols
Receptors, Angiotensin
Trypan Blue
Figure
Reference
-
1. Bayley PJ, Squire LR. Medial temporal lobe amnesia: gradual acquisition of factual information by nondeclarative memory. J Neurosci. 2002; 22:5741–5748.
Article2. Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci. 2007; 18:253–281.
Article3. Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006; 5:139–152.
Article4. Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996; 16:2027–2033.
Article5. Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999; 2:894–897.
Article6. Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. J Comp Neurol. 2006; 495:70–83.
Article7. Rockenstein E, Mante M, Adame A, Crews L, Moessler H, Masliah E. Effects of cerebrolysin™ on neurogenesis in an APP transgenic model of Alzheimer's disease. Acta Neuropathol. 2007; 113:265–275.
Article8. Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer's disease. Neuromolecular Med. 2002; 1:125–135.9. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002; 296:1655–1657.
Article10. Koh SH, Noh MY, Kim SH. Amyloid-beta-induced neurotoxicity is reduced by inhibition of glycogen synthase kinase-3. Brain Res. 2008; 1188:254–262.
Article11. Lee KY, Koh SH, Noh MY, Kim SH, Lee YJ. Phosphatidylinositol-3-kinase activation blocks amyloid beta-induced neurotoxicity. Toxicology. 2008; 243:43–50.
Article12. Noh MY, Koh SH, Kim Y, Kim HY, Cho GW, Kim SH. Neuroprotective effects of donepezil through inhibition of GSK-3 activity in amyloid-beta-induced neuronal cell death. J Neurochem. 2009; 108:1116–1125.
Article13. Brin JP, Anderton BH, Authelet M, Dayanandan R, Leroy K, Lovestone S, et al. Neurofibrillary tangles and tau phosphorylation. Biochem Soc Symp. 2001; 67:81–88.
Article14. Kirschenbaum F, Hsu SC, Cordell B, McCarthy JV. Glycogen synthase kinase 3-beta regulates presenilin 1 C-terminal fragment levels. J Biol Chem. 2001; 276:30701–30707.
Article15. Koh SH, Kim SH, Kwon H, Kim JG, Kim JH, Yang KH, et al. Phosphatidylinositol-3 kinase/Akt and GSK-3 mediated cytoprotective effect of epigallocatechin gallate on oxidative stress-injured neuronal-differentiated N18D3 cells. Neurotoxicology. 2004; 25:793–802.
Article16. Varela-Nallar L, Aranguiz FC, Abbott AC, Slater PG, Inestrosa NC. Adult hippocampal neurogenesis in aging and Alzheimer's disease. Birth Defects Res C Embryo Today. 2010; 90:284–296.
Article17. Callera GE, Antunes TT, Correa JW, Moorman D, Gutsol A, He Y, et al. Differential renal effects of candesartan at high and ultra-high doses in diabetic mice-potential role of the ACE2/AT2R/Mas axis. Biosci Rep. 2016; 36:e00398.
Article18. Kabel AM, Elkhoely AA. Ameliorative effect of Coenzyme Q10 and/or Candesartan on carboplatin-induced nephrotoxicity: roles of apoptosis, transforming growth factor-Β1, nuclear factor kappa-B and the Nrf2/HO-1 pathway. Asian Pac J Cancer Prev. 2017; 18:1629–1636.19. Park CH, Kang JS, Yoon EH, Shim JW, Kim HS, Lee SH. Proneural bHLH neurogenin 2 differentially regulates Nurr1-induced dopamine neuron differentiation in rat and mouse neural precursor cells in vitro. FEBS Lett. 2008; 582:537–542.
Article20. Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Gene Dev. 1996; 10:3129–3140.
Article21. Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002; 277:32046–32053.
Article22. Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005; 8:1051–1058.23. Matsuyama S, Teraoka R, Mori H, Tomiyama T. Inverse correlation between amyloid precursor protein and synaptic plasticity in transgenic mice. Neuroreport. 2007; 18:1083–1087.
Article24. Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004; 24:565–575.
Article25. Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, et al. Intracellular Abeta42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer's disease. FASEB J. 2005; 19:255–257.26. Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004; 82:171–177.
Article27. Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol. 2007; 501:659–667.
Article28. Emam HT, Madboly AG, Shoman AA, Hussein NI. The possible protective role of candesartan on cyclosporine induced nephrotoxicity in rat. Med J Cairo Univ. 2013; 81:271–278.29. Sherif IO, Al-Mutabagani LA, Alnakhli AM, Sobh MA, Mohammed HE. Renoprotective effects of angiotensin receptor blocker and stem cells in acute kidney injury: involvement of inflammatory and apoptotic markers. Exp Biol Med (Maywood). 2015; 240:1572–1579.
Article30. Nishida Y, Takahashi Y, Nakayama T, Soma M, Asai S. Comparative effect of olmesartan and candesartan on lipid metabolism and renal function in patients with hypertension: a retrospective observational study. Cardiovasc Diabetol. 2011; 10:74.
Article31. Luo H, Wang X, Chen C, Wang J, Zou X, Li C, et al. Oxidative stress causes imbalance of renal renin angiotensin system (RAS) components and hypertension in obese Zucker rats. J Am Heart Assoc. 2015; 4:e001559.
Article32. Pap M, Cooper GM. Role of translation initiation factor 2B in control of cell survival by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta signaling pathway. Mol Cell Biol. 2002; 22:578–586.
Article33. Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001; 7:1321–1327.
Article34. Liu S, Liu S, Wang X, Zhou J, Cao Y, Wang F, et al. The PI3K-Akt pathway inhibits senescence and promotes self-renewal of human skin-derived precursors in vitro. Aging Cell. 2011; 10:661–674.
Article35. Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinasedependent signaling. J Biol Chem. 2004; 279:48063–48070.
Article36. Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006; 25:2697–2707.
Article37. Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001; 294:2186–2189.
Article38. Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci U S A. 2006; 103:111–116.
Article39. Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, et al. Pten deletion in adult neural stem⁄progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009; 29:1874–1886.
Article40. Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997; 16:64–67.
Article41. Nelen MR, van Staveren WC, Peeters EA, Hassel MB, Gorlin RJ, Hamm H, et al. Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum Mol Genet. 1997; 6:1383–1387.
Article42. Li J, Simpson L, Takahashi M, Miliaresis C, Myers MP, Tonks N, et al. The PTEN/MMAC1 tumor suppressor induces cell death that is rescued by the AKT/protein kinase B oncogene. Cancer Res. 1998; 58:5667–5672.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Insulin-Like Growth Factor 1 Actions in Developing Brain and the Interaction with Wnt Pathway
- The role of PI3K/AKT pathway and its therapeutic possibility in Alzheimer's disease
- Amyloid-beta oligomers regulate the properties of human neural stem cells through GSK-3beta signaling
- The Role of the PI3K Pathway in the Regeneration of the Damaged Brain by Neural Stem Cells after Cerebral Infarction
- Neural Stem Cell Death Mechanisms Induced by Amyloid Beta