Korean J Radiol.
2019 May;20(5):791-800. 10.3348/kjr.2018.0474.
Comparison of Monoexponential, Biexponential, Stretched-Exponential, and Kurtosis Models of Diffusion-Weighted Imaging in Differentiation of Renal Solid Masses
- Affiliations
-
- 1Department of Radiology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China. danielrau@163.com
- KMID: 2442713
- DOI: http://doi.org/10.3348/kjr.2018.0474
Abstract
OBJECTIVE
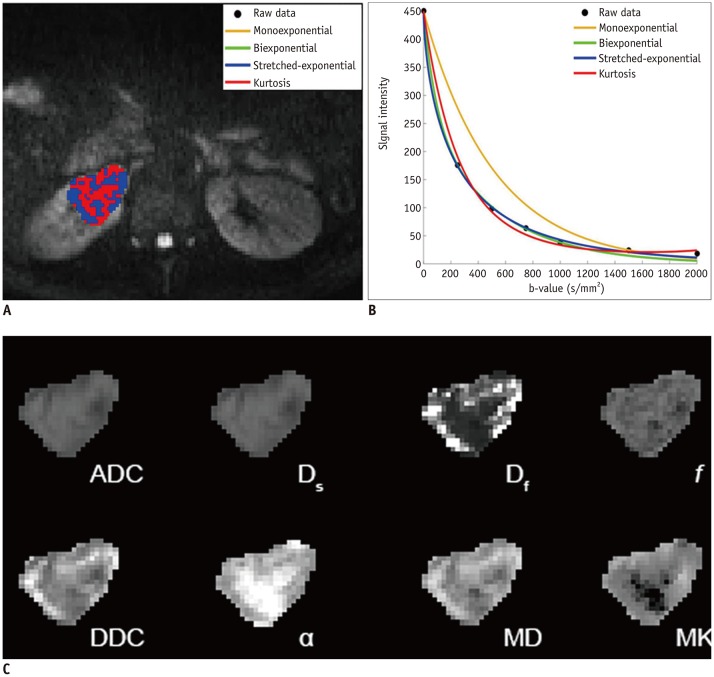
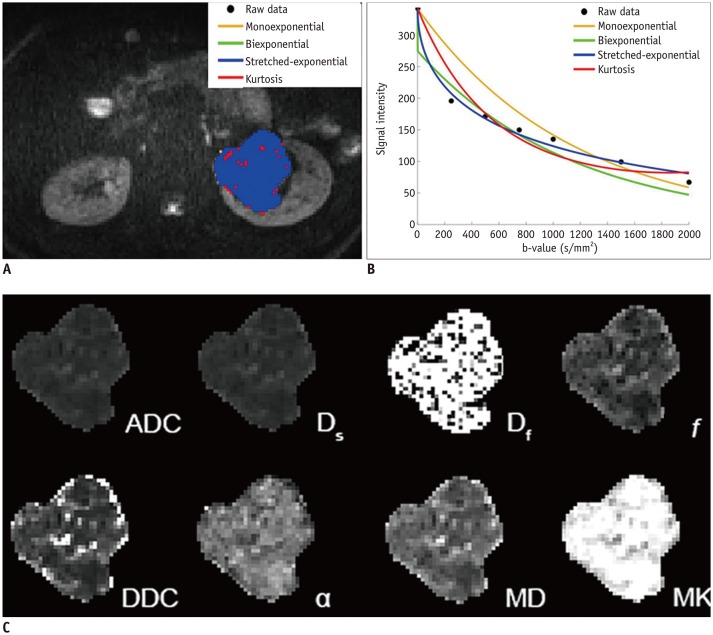
To compare various models of diffusion-weighted imaging including monoexponential apparent diffusion coefficient (ADC), biexponential (fast diffusion coefficient [Df], slow diffusion coefficient [Ds], and fraction of fast diffusion), stretched-exponential (distributed diffusion coefficient and anomalous exponent term [α]), and kurtosis (mean diffusivity and mean kurtosis [MK]) models in the differentiation of renal solid masses.
MATERIALS AND METHODS
A total of 81 patients (56 men and 25 women; mean age, 57 years; age range, 30-69 years) with 18 benign and 63 malignant lesions were imaged using 3T diffusion-weighted MRI. Diffusion model selection was investigated in each lesion using the Akaike information criteria. Mann-Whitney U test and receiver operating characteristic (ROC) analysis were used for statistical evaluations.
RESULTS
Goodness-of-fit analysis showed that the stretched-exponential model had the highest voxel percentages in benign and malignant lesions (90.7% and 51.4%, respectively). ADC, Ds, and MK showed significant differences between benign and malignant lesions (p < 0.05) and between low- and high-grade clear cell renal cell carcinoma (ccRCC) (p < 0.05). α was significantly lower in the benign group than in the malignant group (p < 0.05). All diffusion measures showed significant differences between ccRCC and non-ccRCC (p < 0.05) except Df and α (p = 0.143 and 0.112, respectively). α showed the highest diagnostic accuracy in differentiating benign and malignant lesions with an area under the ROC curve of 0.923, but none of the parameters from these advanced models revealed significantly better performance over ADC in discriminating subtypes or grades of renal cell carcinoma (RCC) (p > 0.05).
CONCLUSION
Compared with conventional diffusion parameters, α may provide additional information for differentiating benign and malignant renal masses, while ADC remains the most valuable parameter for differentiation of RCC subtypes and for ccRCC grading.
Keyword
Figure
Reference
-
1. Sasiwimonphan K, Takahashi N, Leibovich BC, Carter RE, Atwell TD, Kawashima A. Small (<4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology. 2016; 280:653.2. Kutikov A, Fossett LK, Ramchandani P, Tomaszewski JE, Siegelman ES, Banner MP, et al. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology. 2006; 68:737–740. PMID: 17070344.
Article3. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007; 356:125–134. PMID: 17215530.
Article4. Goyal A, Sharma R, Bhalla AS, Gamanagatti S, Seth A, Iyer VK, et al. Diffusion-weighted MRI in renal cell carcinoma: a surrogate marker for predicting nuclear grade and histological subtype. Acta Radiol. 2012; 53:349–358. PMID: 22496427.
Article5. Yu X, Lin M, Ouyang H, Zhou C, Zhang H. Application of ADC measurement in characterization of renal cell carcinomas with different pathological types and grades by 3.0T diffusion-weighted MRI. Eur J Radiol. 2012; 81:3061–3066. PMID: 22651905.
Article6. Wang H, Cheng L, Zhang X, Wang D, Guo A, Gao Y, et al. Renal cell carcinoma: diffusion-weighted MR imaging for subtype differentiation at 3.0T. Radiology. 2010; 257:135–143. PMID: 20713607.7. Zhang J, Tehrani YM, Wang L, Ishill NM, Schwartz LH, Hricak H. Renal masses: characterization with diffusion-weighted MR imaging--a preliminary experience. Radiology. 2008; 247:458–464. PMID: 18430878.
Article8. Sevcenco S, Heinz-Peer G, Ponhold L, Javor D, Kuehhas FE, Klingler HC, et al. Utility and limitations of 3-Tesla diffusion-weighted magnetic resonance imaging for differentiation of renal tumors. Eur J Radiol. 2014; 83:909–913. PMID: 24709332.
Article9. Liu JH, Tian SF, Ju Y, Li Y, Chen AL, Chen LH, et al. Apparent diffusion coefficient measurement by diffusion weighted magnetic resonance imaging is a useful tool in differentiating renal tumors. BMC Cancer. 2015; 15:292. PMID: 25886301.
Article10. Bai Y, Lin Y, Tian J, Shi D, Cheng J, Haacke EM, et al. Grading of gliomas by using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging and diffusion kurtosis MR imaging. Radiology. 2016; 278:496–504. PMID: 26230975.
Article11. Suo S, Cheng F, Cao M, Kang J, Wang M, Hua J, et al. Multiparametric diffusion-weighted imaging in breast lesions: association with pathologic diagnosis and prognostic factors. J Magn Reson Imaging. 2017; 46:740–750. PMID: 28139036.
Article12. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988; 168:497–505. PMID: 3393671.
Article13. Chandarana H, Lee VS, Hecht E, Taouli B, Sigmund EE. Comparison of biexponential and monoexponential model of diffusion weighted imaging in evaluation of renal lesions: preliminary experience. Invest Radiol. 2011; 46:285–291. PMID: 21102345.14. Rheinheimer S, Stieltjes B, Schneider F, Simon D, Pahernik S, Kauczor HU, et al. Investigation of renal lesions by diffusion-weighted magnetic resonance imaging applying intravoxel incoherent motion-derived parameters--initial experience. Eur J Radiol. 2012; 81:e310–e316. PMID: 22104090.
Article15. Bennett KM, Schmainda KM, Bennett RT, Rowe DB, Lu H, Hyde JS. Characterization of continuously distributed cortical water diffusion rates with a stretched-exponential model. Magn Reson Med. 2003; 50:727–734. PMID: 14523958.
Article16. Raab P, Hattingen E, Franz K, Zanella FE, Lanfermann H. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology. 2010; 254:876–881. PMID: 20089718.
Article17. Nogueira L, Brandão S, Matos E, Nunes RG, Loureiro J, Ramos I, et al. Application of the diffusion kurtosis model for the study of breast lesions. Eur Radiol. 2014; 24:1197–1203. PMID: 24658871.
Article18. Rosenkrantz AB, Sigmund EE, Johnson G, Babb JS, Mussi TC, Melamed J, et al. Prostate cancer: feasibility and preliminary experience of a diffusional kurtosis model for detection and assessment of aggressiveness of peripheral zone cancer. Radiology. 2012; 264:126–135. PMID: 22550312.
Article19. Parada Villavicencio C, Mc Carthy RJ, Miller FH. Can diffusion-weighted magnetic resonance imaging of clear cell renal carcinoma predict low from high nuclear grade tumors. Abdom Radiol (NY). 2017; 42:1241–1249. PMID: 27904923.
Article20. Ding Y, Zeng M, Rao S, Chen C, Fu C, Zhou J. Comparison of biexponential and monoexponential model of diffusion-weighted imaging for distinguishing between common renal cell carcinoma and fat poor angiomyolipoma. Korean J Radiol. 2016; 17:853–863. PMID: 27833401.
Article21. Li H, Liang L, Li A, Hu Y, Hu D, Li Z, et al. Monoexponential, biexponential, and stretched exponential diffusion-weighted imaging models: quantitative biomarkers for differentiating renal clear cell carcinoma and minimal fat angiomyolipoma. J Magn Reson Imaging. 2017; 46:240–247. PMID: 27859853.
Article22. Akaike H. [Data analysis by statistical models]. No To Hattatsu. 1992; 24:127–133. PMID: 1567644.23. Ahlawat S, Khandheria P, Del GF, Morelli J, Subhawong TK, Demehri S, et al. Interobserver variability of selective region-of-interest measurement protocols for quantitative diffusion weighted imaging in soft tissue masses: comparison with whole tumor volume measurements. J Magn Reson Imaging. 2016; 43:446–454. PMID: 26174705.
Article24. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.
Article25. Orton MR, Messiou C, Collins D, Morgan VA, Tessier J, Young H, et al. Diffusion-weighted MR imaging of metastatic abdominal and pelvic tumours is sensitive to early changes induced by a VEGF inhibitor using alternative diffusion attenuation models. Eur Radiol. 2016; 26:1412–1419. PMID: 26253255.
Article26. Winfield JM, deSouza NM, Priest AN, Wakefield JC, Hodgkin C, Freeman S, et al. Modelling DW-MRI data from primary and metastatic ovarian tumours. Eur Radiol. 2015; 25:2033–2040. PMID: 25605133.
Article27. Panagiotaki E, Walker-Samuel S, Siow B, Johnson SP, Rajkumar V, Pedley RB, et al. Noninvasive quantification of solid tumor microstructure using VERDICT MRI. Cancer Res. 2014; 74:1902–1912. PMID: 24491802.
Article28. Kim S, Jain M, Harris AB, Lee VS, Babb JS, Sigmund EE, et al. T1 hyperintense renal lesions: characterization with diffusion-weighted MR imaging versus contrast-enhanced MR imaging. Radiology. 2009; 251:796–807. PMID: 19380690.
Article29. Taouli B, Thakur RK, Mannelli L, Babb JS, Kim S, Hecht EM, et al. Renal lesions: characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology. 2009; 251:398–407. PMID: 19276322.
Article30. Shen L, Zhou L, Liu X, Yang X. Comparison of biexponential and monoexponential DWI in evaluation of Fuhrman grading of clear cell renal cell carcinoma. Diagn Interv Radiol. 2017; 23:100–105. PMID: 28050950.
Article31. Chandarana H, Kang SK, Wong S, Rusinek H, Zhang JL, Arizono S, et al. Diffusion-weighted intravoxel incoherent motion imaging of renal tumors with histopathologic correlation. Invest Radiol. 2012; 47:688–696. PMID: 22996315.
Article32. Hectors SJ, Semaan S, Song C, Lewis S, Haines GK, Tewari A, et al. Advanced diffusion-weighted imaging modeling for prostate cancer characterization: correlation with quantitative histopathologic tumor tissue composition-a hypothesis-generating study. Radiology. 2018; 286:918–928. PMID: 29117481.33. Zhu L, Pan Z, Ma Q, Yang W, Shi H, Fu C, et al. Diffusion kurtosis imaging study of rectal adenocarcinoma associated with histopathologic prognostic factors: preliminary findings. Radiology. 2017; 284:66–76. PMID: 27929929.34. Fujima N, Sakashita T, Homma A, Shimizu Y, Yoshida A, Harada T, et al. Advanced diffusion models in head and neck squamous cell carcinoma patients: goodness of fit, relationships among diffusion parameters and comparison with dynamic contrast-enhanced perfusion. Magn Reson Imaging. 2017; 36:16–23. PMID: 27989906.
Article35. Anderson SW, Barry B, Soto J, Ozonoff A, O'Brien M, Jara H. Characterizing non-gaussian, high b-value diffusion in liver fibrosis: stretched exponential and diffusional kurtosis modeling. J Magn Reson Imaging. 2014; 39:827–834. PMID: 24259401.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Utility of Readout-Segmented Echo-Planar Imaging-Based Diffusion Kurtosis Imaging for Differentiating Malignant from Benign Masses in Head and Neck Region
- Biexponential Apparent Diffusion Coefficients Values in the Prostate: Comparison among Normal Tissue, Prostate Cancer, Benign Prostatic Hyperplasia and Prostatitis
- RE: Distinguishing between Renal Cell Carcinoma and Fat Poor Angiomyolipoma in Diffusion-Weighted Imaging
- Adnexal Masses: Clinical Application of Multiparametric MR Imaging & O-RADS MRI
- Incidental Solid Renal Masses: Radiologic Assessment and Managements