Korean J Radiol.
2013 Apr;14(2):222-232. 10.3348/kjr.2013.14.2.222.
Biexponential Apparent Diffusion Coefficients Values in the Prostate: Comparison among Normal Tissue, Prostate Cancer, Benign Prostatic Hyperplasia and Prostatitis
- Affiliations
-
- 1Department of Radiology, Fudan University Shanghai Cancer Center, Shanghai 200032, China. pengweijun2010@126.com
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China.
- 3Global Applied Science Laboratory, GE Healthcare, Shanghai 201203, China.
- KMID: 1482781
- DOI: http://doi.org/10.3348/kjr.2013.14.2.222
Abstract
OBJECTIVE
To investigate the biexponential apparent diffusion parameters of diverse prostate tissues and compare them with monoexponential apparent diffusion coefficient (ADC) value in the efficacy to discriminate prostate cancer from benign lesions.
MATERIALS AND METHODS
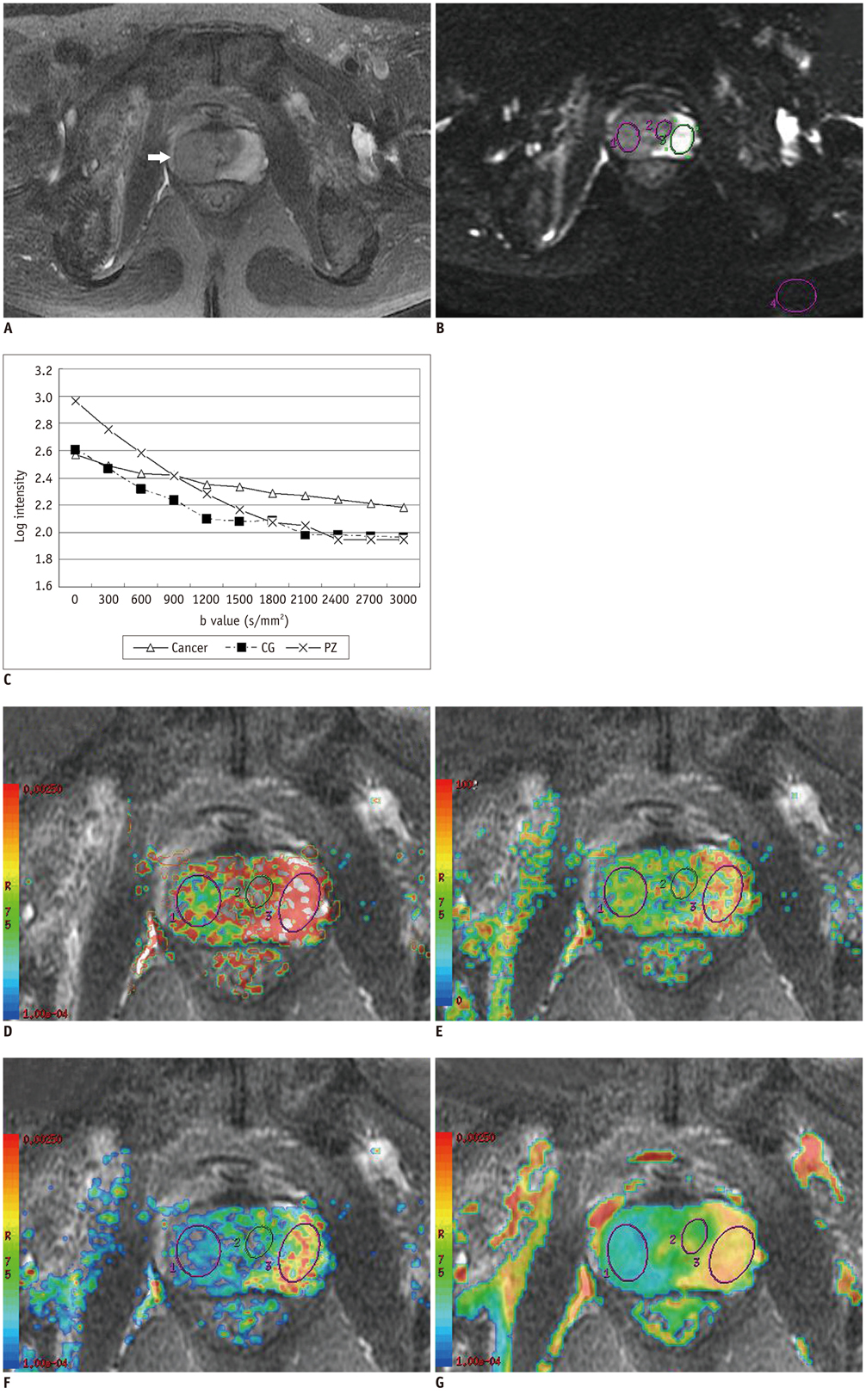
Eleven healthy volunteers and 61 patients underwent a conventional (b-factors 0, 1000 s/mm2) and a 10 b-factor (0 to 3000 s/mm2) diffusion-weighted imaging (DWI). The monoexponential ADC value and biexponential parameters of fast ADC (ADCf), fraction of ADCf (f), slow ADC (ADCs) value for 29 prostate cancer, 28 benign prostatic hyperplasia (BPH), 24 prostatitis lesions and normal tissue were calculated and compared. Receiver operating characteristic analysis was performed to determine the sensitivity, specificity and optimal cut-off points.
RESULTS
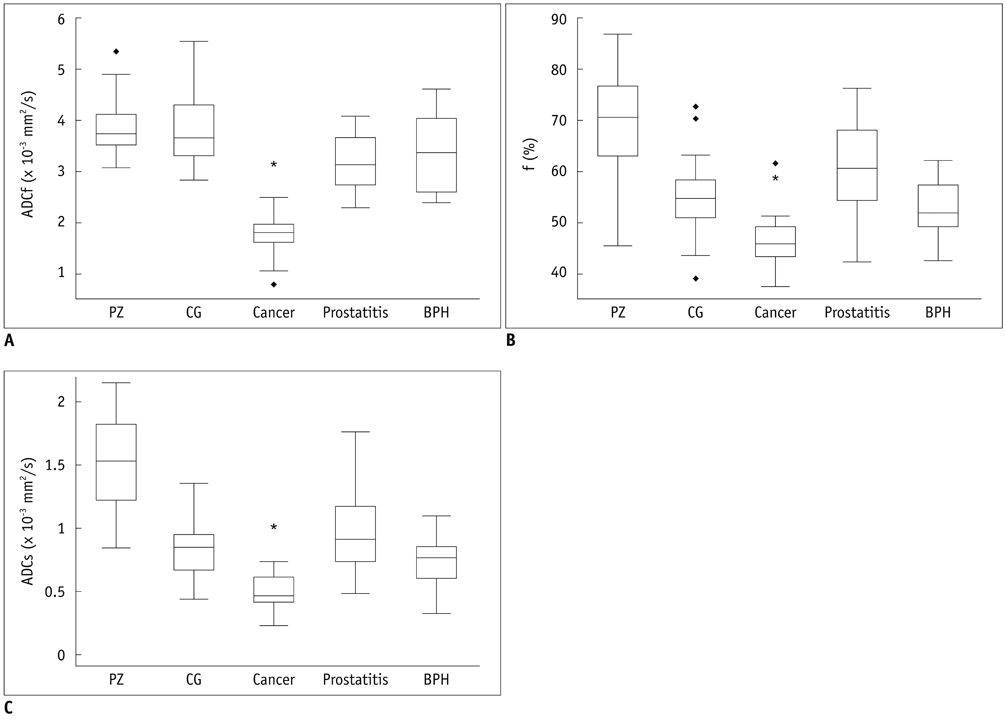
Prostate cancer had lower ADC, ADCf, f, and ADCs than all other tissues (p < 0.01). Prostatitis exhibited a lower ADC, ADCf, ADCs and f than the peripheral zone tissue (p < 0.01), and BPH showed a lower ADC and ADCf than the central gland tissue (p < 0.01). The ADCf demonstrated a comparable accuracy with ADC in differentiating cancer from BPH [area under the curve (AUC) 0.93 vs. 0.92] and prostatitis AUC 0.98 vs. 0.99) (both p > 0.05), but the AUC of f and ADCs in differentiating cancer from BPH (0.73 and 0.81) and prostatitis (0.88 and 0.91) were significantly lower than ADC (all p < 0.05).
CONCLUSION
The biexponential DWI appears to provide additional parameters for tissue characterization in prostate, and ADCf helps to yield comparable accuracy with ADC in differentiating cancer from benign lesions.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Analysis of Variance
Biopsy
Diagnosis, Differential
*Diffusion Magnetic Resonance Imaging
Humans
Male
Middle Aged
Prospective Studies
Prostate-Specific Antigen/blood
Prostatectomy
Prostatic Hyperplasia/*diagnosis
Prostatic Neoplasms/*diagnosis/surgery
Prostatitis/*diagnosis
ROC Curve
Sensitivity and Specificity
Prostate-Specific Antigen
Figure
Reference
-
1. Cancer Registration Committee of the Japanese Urological Association. Clinicopathological statistics on registered prostate cancer patients in Japan: 2000 report from the Japanese Urological Association. Int J Urol. 2005. 12:46–61.2. Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part II: individual countries. BJU Int. 2002. 90:174–184.3. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999. 80:827–841.4. Wilkinson BA, Hamdy FC. State-of-the-art staging in prostate cancer. BJU Int. 2001. 87:423–430.5. Quint LE, Van Erp JS, Bland PH, Del Buono EA, Mandell SH, Grossman HB, et al. Prostate cancer: correlation of MR images with tissue optical density at pathologic examination. Radiology. 1991. 179:837–842.6. Ikonen S, Kivisaari L, Tervahartiala P, Vehmas T, Taari K, Rannikko S. Prostatic MR imaging. Accuracy in differentiating cancer from other prostatic disorders. Acta Radiol. 2001. 42:348–354.7. Kim JK, Jang YJ, Cho G. Multidisciplinary functional MR imaging for prostate cancer. Korean J Radiol. 2009. 10:535–551.8. Miao H, Fukatsu H, Ishigaki T. Prostate cancer detection with 3-T MRI: comparison of diffusion-weighted and T2-weighted imaging. Eur J Radiol. 2007. 61:297–302.9. Kim CK, Park BK, Lee HM, Kwon GY. Value of diffusion-weighted imaging for the prediction of prostate cancer location at 3T using a phased-array coil: preliminary results. Invest Radiol. 2007. 42:842–847.10. Jung DC, Lee HJ, Seo JW, Park SY, Lee SJ, Lee JH, et al. Diffusion-weighted imaging of a prostate cancer xenograft model seen on a 7 tesla animal MR scanner: comparison of ADC values and pathologic findings. Korean J Radiol. 2012. 13:82–89.11. Mulkern RV, Vajapeyam S, Robertson RL, Caruso PA, Rivkin MJ, Maier SE. Biexponential apparent diffusion coefficient parametrization in adult vs newborn brain. Magn Reson Imaging. 2001. 19:659–668.12. Niendorf T, Dijkhuizen RM, Norris DG, van Lookeren Campagne M, Nicolay K. Biexponential diffusion attenuation in various states of brain tissue: implications for diffusion-weighted imaging. Magn Reson Med. 1996. 36:847–857.13. Maier SE, Bogner P, Bajzik G, Mamata H, Mamata Y, Repa I, et al. Normal brain and brain tumor: multicomponent apparent diffusion coefficient line scan imaging. Radiology. 2001. 219:842–849.14. Brugières P, Thomas P, Maraval A, Hosseini H, Combes C, Chafiq A, et al. Water diffusion compartmentation at high b values in ischemic human brain. AJNR Am J Neuroradiol. 2004. 25:692–698.15. Mulkern RV, Barnes AS, Haker SJ, Hung YP, Rybicki FJ, Maier SE, et al. Biexponential characterization of prostate tissue water diffusion decay curves over an extended b-factor range. Magn Reson Imaging. 2006. 24:563–568.16. Shinmoto H, Oshio K, Tanimoto A, Higuchi N, Okuda S, Kuribayashi S, et al. Biexponential apparent diffusion coefficients in prostate cancer. Magn Reson Imaging. 2009. 27:355–359.17. Villeirs GM, De Meerleer GO. Magnetic resonance imaging (MRI) anatomy of the prostate and application of MRI in radiotherapy planning. Eur J Radiol. 2007. 63:361–368.18. Gilbert G. Measurement of signal-to-noise ratios in sum-of-squares MR images. J Magn Reson Imaging. 2007. 26:1678. author reply 1679.19. Ren J, Huan Y, Wang H, Zhao H, Ge Y, Chang Y, et al. Diffusion-weighted imaging in normal prostate and differential diagnosis of prostate diseases. Abdom Imaging. 2008. 33:724–728.20. Le Bihan D. The 'wet mind': water and functional neuroimaging. Phys Med Biol. 2007. 52:R57–R90.21. McNeal JE. Normal histology of the prostate. Am J Surg Pathol. 1988. 12:619–633.22. Nickel JC, True LD, Krieger JN, Berger RE, Boag AH, Young ID. Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int. 2001. 87:797–805.23. Chagas MA, Babinski MA, Costa WS, Sampaio FJ. Stromal and acinar components of the transition zone in normal and hyperplastic human prostate. BJU Int. 2002. 89:699–702.24. Tamura T, Usui S, Murakami S, Arihiro K, Fujimoto T, Yamada T, et al. Comparisons of multi b-value DWI signal analysis with pathological specimen of breast cancer. Magn Reson Med. 2011. [Epub ahead of print].25. Riches SF, Hawtin K, Charles-Edwards EM, de Souza NM. Diffusion-weighted imaging of the prostate and rectal wall: comparison of biexponential and monoexponential modelled diffusion and associated perfusion coefficients. NMR Biomed. 2009. 22:318–325.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The role of serum prostate specific antigen in prostatic cancer and benign prostatic hyperplasia
- Prostatic Disease and Sexual Dysfunction
- The Effects of Concomitant Prostatic Calculi to the Therapeutic Results in Patients with Chronic Bacterial Prostatitis
- Malacoplakia of the Prostate
- Inflammation of Prostate and Prostate-Specific Antigen