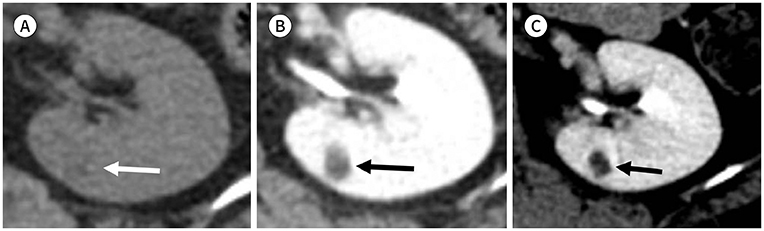
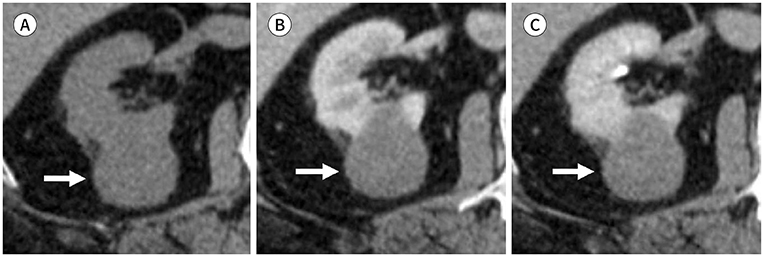
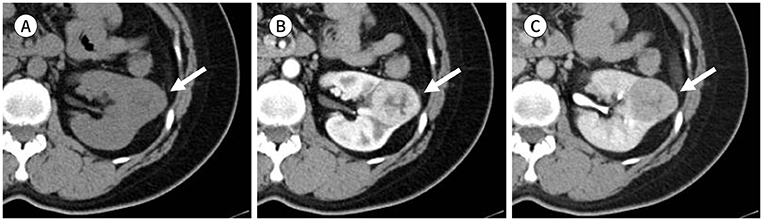
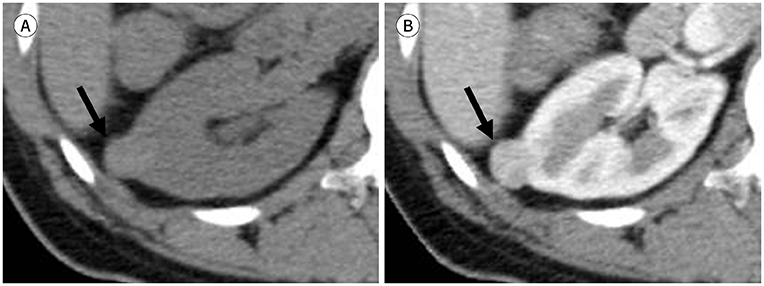
J Korean Soc Radiol.
2019 Nov;80(6):1010-1029. 10.3348/jksr.2019.80.6.1010.
Incidental Solid Renal Masses: Radiologic Assessment and Managements
- Affiliations
-
- 1Department of Radiology, Dong-A University College of Medicine, Busan, Korea. kimdw@dau.ac.kr
- KMID: 2464904
- DOI: http://doi.org/10.3348/jksr.2019.80.6.1010
Abstract
- The steady increase in imaging studies has led to the incidental discovery of many renal masses. Although most incidental solid renal masses are asymptomatic and small in size, they are mostly malignant renal cancers necessitating accurate diagnosis. Small-sized masses are more likely to be benign tumors; therefore, access is needed according to size. Because most malignant tumors are renal cell carcinoma, and most benign tumors are angiomyolipoma and oncocytoma. Knowledge of common imaging findings of these tumors is helpful for diagnosis and management. However, imaging techniques are often insufficient to characterize solid renal masses which are discovered incidentally in radiological examinations, especially small-sized masses. Herein, we describe the imaging features characteristic of incidental solid renal masses and discuss their management in cases when an accurate diagnosis could or could not be made.
MeSH Terms
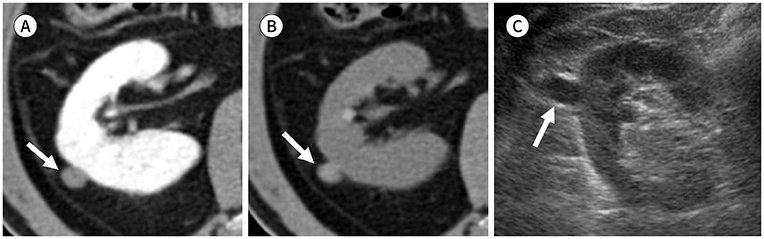
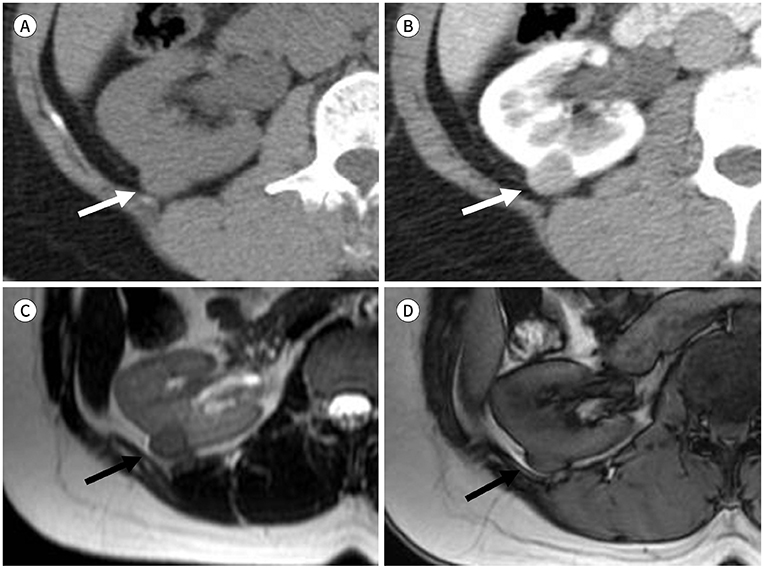
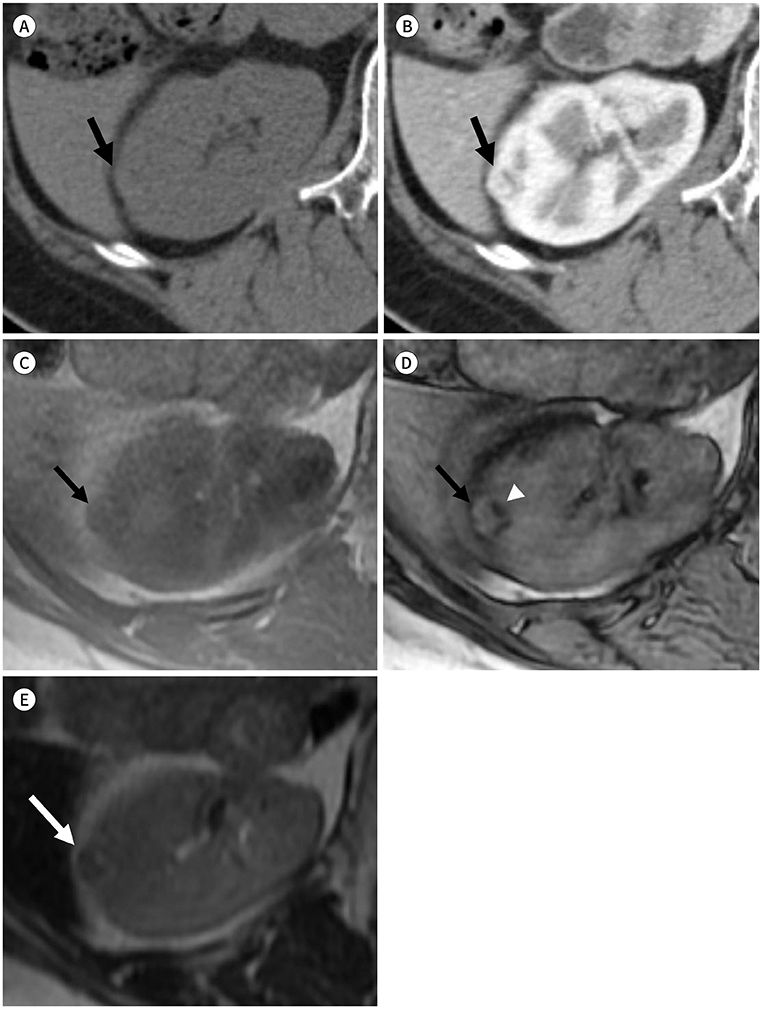
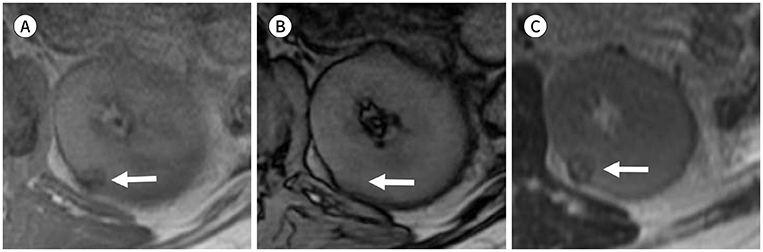
Figure
Reference
-
1. Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998; 51:203–205.2. Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003; 58:626–629.3. Kay FU, Pedrosa I. Imaging of solid renal masses. Radiol Clin North Am. 2017; 55:243–258.4. Silverman SG, Israel GM, Trinh QD. Incompletely characterized incidental renal masses: emerging data support conservative management. Radiology. 2015; 275:28–42.5. Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer. 2004; 100:738–745.6. Bradley AJ, Lim YY, Singh FM. Imaging features, follow-up, and management of incidentally detected renal lesions. Clin Radiol. 2011; 66:1129–1139.7. Woo S, Cho JY. Imaging findings of common benign renal tumors in the era of small renal masses: differential diagnosis from small renal cell carcinoma: current status and future perspectives. Korean J Radiol. 2015; 16:99–113.8. Sasaguri K, Takahashi N. CT and MR imaging for solid renal mass characterization. Eur J Radiol. 2018; 99:40–54.9. Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, et al. Kidney cancer, version 2.2017, NCCN Clinical Practice Guidelines in oncology. J Natl Compr Canc Netw. 2017; 15:804–834.10. Finelli A, Ismaila N, Bro B, Durack J, Eggener S, Evans A, et al. Management of small renal masses: American Society of clinical oncology clinical practice guideline. J Clin Oncol. 2017; 35:668–680.11. Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017; 198:520–529.12. Herts BR, Silverman SG, Hindman NM, Uzzo RG, Hartman RP, Israel GM, et al. Management of the incidental renal mass on CT: a white paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2018; 15:264–273.13. Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y. The natural history of simple renal cysts. J Urol. 2002; 167:21–23.14. Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer. 2000; 89:604–614.15. Bosniak MA. Diagnosis and management of patients with complicated cystic lesions of the kidney. AJR Am J Roentgenol. 1997; 169:819–821.16. Umbreit EC, Shimko MS, Childs MA, Lohse CM, Cheville JC, Leibovich BC, et al. Metastatic potential of a renal mass according to original tumour size at presentation. BJU Int. 2012; 109:190–194. discussion 194.17. Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003; 170:2217–2220.18. Thompson RH, Hill JR, Babayev Y, Cronin A, Kaag M, Kundu S, et al. Metastatic renal cell carcinoma risk according to tumor size. J Urol. 2009; 182:41–45.19. Simpson E, Patel U. Diagnosis of angiomyolipoma using computed tomography-region of interest < or =−10 HU or 4 adjacent pixels < or =−10 HU are recommended as the diagnostic thresholds. Clin Radiol. 2006; 61:410–416.20. Smaldone MC, Kutikov A, Egleston BL, Canter DJ, Viterbo R, Chen DY, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. 2012; 118:997–1006.21. Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006; 175:425–431.22. Patel N, Cranston D, Akhtar MZ, George C, Jones A, Leiblich A, et al. Active surveillance of small renal masses offers short-term oncological efficacy equivalent to radical and partial nephrectomy. BJU Int. 2012; 110:1270–1275.23. Jamis-Dow CA, Choyke PL, Jennings SB, Linehan WM, Thakore KN, Walther MM. Small (< or = 3-cm) renal masses: detection with CT versus US and pathologic correlation. Radiology. 1996; 198:785–778.24. Jinzaki M, Tanimoto A, Narimatsu Y, Ohkuma K, Kurata T, Shinmoto H, et al. Angiomyolipoma: imaging findings in lesions with minimal fat. Radiology. 1997; 205:497–502.25. Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. 2014; 39:588–604.26. Siegel CL, Middleton WD, Teefey SA, McClennan BL. Angiomyolipoma and renal cell carcinoma: US differentiation. Radiology. 1996; 198:789–793.27. Atri M, Tabatabaeifar L, Jang HJ, Finelli A, Moshonov H, Jewett M. Accuracy of contrast-enhanced US for differentiating benign from malignant solid small renal masses. Radiology. 2015; 276:900–908.28. Jonisch AI, Rubinowitz AN, Mutalik PG, Israel GM. Can high-attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced CT? Radiology. 2007; 243:445–450.29. Birnbaum BA, Hindman N, Lee J, Babb JS. Renal cyst pseudoenhancement: influence of multidetector CT reconstruction algorithm and scanner type in phantom model. Radiology. 2007; 244:767–775.30. Maki DD, Birnbaum BA, Chakraborty DP, Jacobs JE, Carvalho BM, Herman GT. Renal cyst pseudoenhancement: beam-hardening effects on CT numbers. Radiology. 1999; 213:468–472.31. Krajewski KM, Shinagare AB. Novel imaging in renal cell carcinoma. Curr Opin Urol. 2016; 26:388–395.32. Silverman SG, Mortele KJ, Tuncali K, Jinzaki M, Cibas ES. Hyperattenuating renal masses: etiologies, pathogenesis, and imaging evaluation. Radiographics. 2007; 27:1131–1143.33. Goiney RC, Goldenberg L, Cooperberg PL, Charboneau JW, Rosenfield AT, Russin LD, et al. Renal oncocytoma: sonographic analysis of 14 cases. AJR Am J Roentgenol. 1984; 143:1001–1004.34. Woo S, Cho JY, Kim SH, Kim SY. Comparison of segmental enhancement inversion on biphasic MDCT between small renal oncocytomas and chromophobe renal cell carcinomas. AJR Am J Roentgenol. 2013; 201:598–604.35. Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology. 2002; 225:78–82.36. Woo S, Cho JY, Kim SH, Kim SY. Angiomyolipoma with minimal fat and non-clear cell renal cell carcinoma: differentiation on MDCT using classification and regression tree analysis-based algorithm. Acta Radiol. 2014; 55:1258–1269.37. Wasser EJ, Shyn PB, Riveros-Angel M, Sadow CA, Steele GS, Silverman SG. Renal cell carcinoma containing abundant non-calcified fat. Abdom Imaging. 2013; 38:598–602.38. Kreft BP, Müller-Miny H, Sommer T, Steudel A, Vahlensieck M, Novak D, et al. Diagnostic value of MR imaging in comparison to CT in the detection and differential diagnosis of renal masses: ROC analysis. Eur Radiol. 1997; 7:542–547.39. Pedrosa I, Sun MR, Spencer M, Genega EM, Olumi AF, Dewolf WC, et al. MR imaging of renal masses: correlation with findings at surgery and pathologic analysis. Radiographics. 2008; 28:985–1003.40. Israel GM, Hindman N, Hecht E, Krinsky G. The use of opposed phase chemical shift MRI in the diagnosis of renal angiomyolipomas. AJR Am J Roentgenol. 2005; 184:1868–1872.41. Hakim SW, Schieda N, Hodgdon T, McInnes MD, Dilauro M, Flood TA. Angiomyolipoma (AML) without visible fat: Ultrasound, CT and MR imaging features with pathological correlation. Eur Radiol. 2016; 26:592–600.42. Childs DD, Clingan MJ, Zagoria RJ, Sirintrapun J, Tangtiang K, Anderson A, et al. In-phase signal intensity loss in solid renal masses on dual-echo gradient-echo MRI: association with malignancy and pathologic classification. AJR Am J Roentgenol. 2014; 203:W421–W428.43. Lassel EA, Rao R, Schwenke C, Schoenberg SO, Michaely HJ. Diffusion-weighted imaging of focal renal lesions: a meta-analysis. Eur Radiol. 2014; 24:241–249.44. Campbell N, Rosenkrantz AB, Pedrosa I. MRI phenotype in renal cancer: is it clinically relevant? Top Magn Reson Imaging. 2014; 23:95–115.45. Cornelis F, Tricaud E, Lasserre AS, Petitpierre F, Bernhard JC, Le Bras Y, et al. Multiparametric magnetic resonance imaging for the differentiation of low and high grade clear cell renal carcinoma. Eur Radiol. 2014; 25:24–31.46. Silverman SG, Israel GM, Herts BR, Richie JP. Management of the incidental renal mass. Radiology. 2008; 249:16–31.47. Patel HD, Johnson MH, Pierorazio PM, Sozio SM, Sharma R, Iyoha E, et al. Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: systematic review of the literature. J Urol. 2016; 195:1340–1347.48. Silverman SG, Gan YU, Mortele KJ, Tuncali K, Cibas ES. Renal masses in the adult patient: the role of percutaneous biopsy. Radiology. 2006; 240:6–22.49. Rybicki FJ, Shu KM, Cibas ES, Fielding JR, VanSonnenberg E, Silverman SG. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003; 180:1281–1287.50. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009; 9:44–55.51. Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006; 7:735–740.52. Huang WC, Atoria CL, Bjurlin M, Pinheiro LC, Russo P, Lowrance WT, et al. Management of small kidney cancers in the new millennium: contemporary trends and outcomes in a population-based cohort. JAMA Surg. 2015; 150:664–672.53. Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008; 179:468–471. discussion 472-473.54. Schmit GD, Schenck LA, Thompson RH, Boorjian SA, Kurup AN, Weisbrod AJ, et al. Predicting renal cryoablation complications: new risk score based on tumor size and location and patient history. Radiology. 2014; 272:903–910.55. Haramis G, Mues AC, Rosales JC, Okhunov Z, Lanzac AP, Badani K, et al. Natural history of renal cortical neoplasms during active surveillance with follow-up longer than 5 years. Urology. 2011; 77:787–791.56. Organ M, Jewett M, Basiuk J, Morash C, Pautler S, Siemens DR, et al. Growth kinetics of small renal masses: a prospective analysis from the Renal Cell Carcinoma Consortium of Canada. Can Urol Assoc J. 2014; 8:24–27.57. Kurup AN, Thompson RH, Leibovich BC, Harmsen WS, Sebo TJ, Callstrom MR, et al. Renal oncocytoma growth rates before intervention. BJU Int. 2012; 110:1444–1448.58. Remzi M, Ozsoy M, Klingler HC, Susani M, Waldert M, Seitz C, et al. Are small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameter. J Urol. 2006; 176:896–889.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection and Identification of Renal Masses by Abdominal Ultrasonography and Incidence Estimation
- The Comparison between Pre- and Postoperative Diagnosis in Renal Masses Smaller than 3cm in Diameter
- Detection Rate of Breast Lesion on Mammogram Shown on Breast Sonogram
- Ultrasonography of ovarian masses using a pattern recognition approach
- Characteristics of Incidentally Detected Renal Cell Carcinoma