Infect Chemother.
2019 Mar;51(1):10-20. 10.3947/ic.2019.51.1.10.
Can Aminoglycosides Be Used as a New Treatment for Helicobacter pylori? In vitro Activity of Recently Isolated Helicobacter pylori
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. imfell@yuhs.ac
- 2Division of Gastroenterology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Korea Institute of Geoscience and Mineral Resources, Daejeon, Korea. imkang@kigam.re.kr
- KMID: 2442217
- DOI: http://doi.org/10.3947/ic.2019.51.1.10
Abstract
- BACKGROUND
Smectite can serve as a drug delivery system and gentamicin-intercalated smectite hybrids are expected to supersede the standard therapy for Helicobacter pylori eradication. The aim of this study was to confirm whether the minimum inhibitory concentration (MIC) of aminoglycosides applied as smectite hybrids remained low against recently isolated H. pylori strains.
MATERIALS AND METHODS
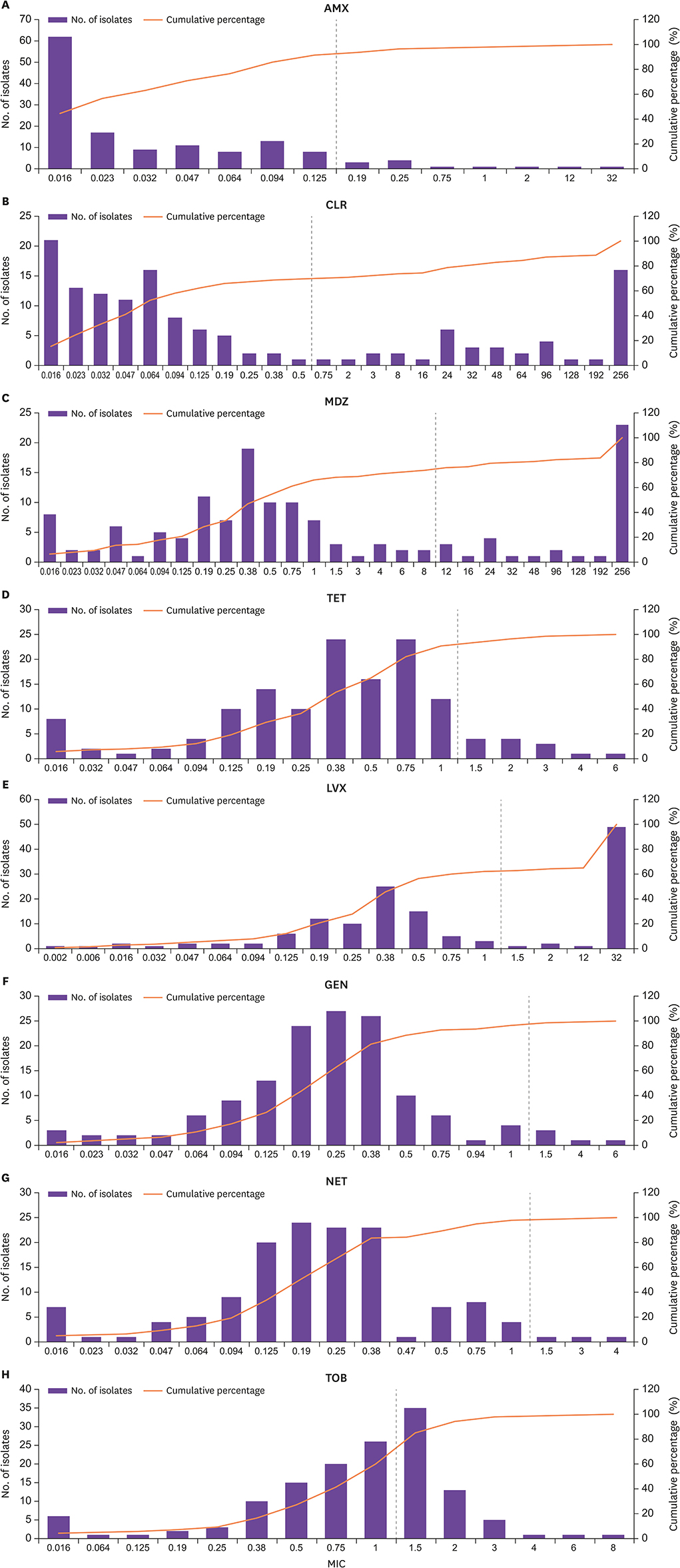
A total of 140 strains were collected for a minimum period of 3 years. Antimicrobial susceptibility tests were performed, and the MICs of eight antibiotics (amoxicillin, clarithromycin, metronidazole, tetracycline, levofloxacin, gentamicin, netilmicin, and tobramycin) were determined by using the Epsilometer test and following the European Committee on Antimicrobial Susceptibility Testing recommendations.
RESULTS
The resistance rate of clarithromycin was high, up to 30.7%, although it is a major antimicrobial agent used in standard therapy. The MIC50 and MIC90 of gentamicin (0.25 mg/L and 0.75 mg/L) and netilmicin (0.19 mg/L and 0.75 mg/L) were lower than other alternative therapies for H. pylori eradication. In clarithromycin-resistant strains, the MIC50 was 0.25 mg/L and the MIC90 was 1 mg/L for gentamicin; for netilmicin, the values were 0.25 mg/L and 0.75 mg/L, respectively.
CONCLUSION
Through the use of gentamicin and netilmicin, which have low MICs for H. pylori, aminoglycoside-intercalated smectite hybrids are expected to emerge as a new standard therapy for H. pylori eradication.
Keyword
MeSH Terms
-
Aminoglycosides*
Anti-Bacterial Agents
Clarithromycin
Complementary Therapies
Disk Diffusion Antimicrobial Tests
Drug Delivery Systems
Gentamicins
Helicobacter pylori*
Helicobacter*
In Vitro Techniques*
Levofloxacin
Metronidazole
Microbial Sensitivity Tests
Netilmicin
Tetracycline
Aminoglycosides
Anti-Bacterial Agents
Clarithromycin
Gentamicins
Metronidazole
Netilmicin
Tetracycline
Figure
Reference
-
1. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FK, Sung JJY, Kaplan GG, Ng SC. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017; 153:420–429.
Article2. Yim JY, Kim N, Choi SH, Kim YS, Cho KR, Kim SS, Seo GS, Kim HU, Baik GH, Sin CS, Cho SH, Oh BH. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007; 12:333–340.
Article3. Lim SH, Kwon JW, Kim N, Kim GH, Kang JM, Park MJ, Yim JY, Kim HU, Baik GH, Seo GS, Shin JE, Joo YE, Kim JS, Jung HC. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013; 13:104.
Article4. Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001; 345:784–789.
Article5. Seta T, Takahashi Y, Noguchi Y, Shikata S, Sakai T, Sakai K, Yamashita Y, Nakayama T. Effectiveness of Helicobacter pylori eradication in the prevention of primary gastric cancer in healthy asymptomatic people: a systematic review and meta-analysis comparing risk ratio with risk difference. PLoS One. 2017; 12:e0183321.6. Heo J, Jeon SW. Changes in the eradication rate of conventional triple therapy for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2014; 63:141–145.
Article7. Kim SE, Park MI, Park SJ, Moon W, Choi YJ, Cheon JH, Kwon HJ, Ku KH, Yoo CH, Kim JH, Lee GW, Song SE. Trends in Helicobacter pylori eradication rates by first-line triple therapy and related factors in eradication therapy. Korean J Intern Med. 2015; 30:801–807.
Article8. Lee JH, Shin JH, Roe IH, Sohn SG, Lee JH, Kang GH, Lee HK, Jeong BC, Lee SH. Impact of clarithromycin resistance on eradication of Helicobacter pylori in infected adults. Antimicrob Agents Chemother. 2005; 49:1600–1603.
Article9. Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010; 8:59–70.
Article10. Gasparetto M, Pescarin M, Guariso G. Helicobacter pylori eradication therapy: current availabilities. ISRN Gastroenterol. 2012; 2012:186734.11. Shin WG, Lee SW, Baik GH, Huh KC, Lee SI, Chung JW, Jung WT, Park MI, Jung HK, Kim HU, Kim JH, Seol SY, Yoon SM, Jeon SW, Hong SJ, Kim GH, Lee DH, Kim HS, Choi SC, Kang HM, Lee J, Kim JG, Kim JJ. Eradication rates of Helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: nationwide survey. Helicobacter. 2016; 21:266–278.
Article12. Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American college of gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007; 102:1808–1825.
Article13. Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013; 88:33–45.
Article14. Kim SG, Jung HK, Lee HL, Jang JY, Lee H, Kim CG, Shin WG, Shin ES, Lee YC. Korean College of Helicobacter and Upper Gastrointestinal Research. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol. 2014; 29:1371–1386.
Article15. Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014; 12:177–86.e3. Discussion e12-3.
Article16. Sugimoto M, Furuta T. Efficacy of tailored Helicobacter pylori eradication therapy based on antibiotic susceptibility and CYP2C19 genotype. World J Gastroenterol. 2014; 20:6400–6411.
Article17. Draeger S, Wüppenhorst N, Kist M, Glocker EO. Outcome of second- and third-line Helicobacter pylori eradication therapies based on antimicrobial susceptibility testing. J Antimicrob Chemother. 2015; 70:3141–3145.
Article18. Hu Y, Zhang M, Lu B, Dai J. Helicobacter pylori and antibiotic resistance, a continuing and intractable problem. Helicobacter. 2016; 21:349–363.
Article19. Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P, Marshall JK. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016; 151:51–69.e14.
Article20. Kumana CR, Yuen KY. Parenteral aminoglycoside therapy. Selection, administration and monitoring. Drugs. 1994; 47:902–913.21. Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008; 134:306–323.
Article22. Jeong SJ, Kim JH, Jung DH, Lee KH, Park SY, Song Y, Kang IM, Song YG. Gentamicin-intercalated smectite as a new therapeutic option for Helicobacter pylori eradication. J Antimicrob Chemother. 2018; 73:1324–1329.23. Irie Y, Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Antibiotic MICs and short time-killing against Helicobacter pylori: therapeutic potential of kanamycin. J Antimicrob Chemother. 1997; 40:235–240.
Article24. Brenciaglia MI, Fornara AM, Scaltrito MM, Braga PC, Dubini F. Activity of amoxicillin, metronidazole, bismuth salicylate and six aminoglycosides against Helicobacter pylori . J Chemother. 1996; 8:52–54.
Article25. Valdez Y, Velapatiño B, Gilman RH, Gutierrez V, León C. Antimicrobial susceptibility of Helicobacter pylori determined by the E test using tetrazolium egg yolk agar. J Clin Microbiol. 1998; 36:2784–2785.
Article26. Piccolomini R, Di Bonaventura G, Catamo G, Carbone F, Neri M. Comparative evaluation of the E test, agar dilution, and broth microdilution for testing susceptibilities of Helicobacter pylori strains to 20 antimicrobial agents. J Clin Microbiol. 1997; 35:1842–1846.
Article27. European Committee on Antimicrobial Susceptibility Testing. Breakpoint-bacteria (v 8.0). Accessed 2018 March 12. Available at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdf.28. Hosaka Y, Irinoda K, Nakano R, Kitasato H, Okamoto R, Saigenji K, Inoue M. Antibacterial activity of 16 antibiotics against Helicobacter pylori . Jpn J Antibiot. 2000; 53:623–630.29. Wang C, Ding Y, Teppen BJ, Boyd SA, Song C, Li H. Role of interlayer hydration in lincomycin sorption by smectite clays. Environ Sci Technol. 2009; 43:6171–6176.
Article30. Li Z, Chang PH, Jean JS, Jiang WT, Wang CJ. Interaction between tetracycline and smectite in aqueous solution. J Colloid Interface Sci. 2010; 341:311–319.
Article31. Trivedi V, Nandi U, Maniruzzaman M, Coleman NJ. Intercalated theophylline-smectite hybrid for pH-mediated delivery. Drug Deliv Transl Res. 2018; 8:1781–1789.
Article32. Nanavaty J, Mortensen JE, Shryock TR. The effects of environmental conditions on the in vitro activity of selected antimicrobial agents against Escherichia coli . Curr Microbiol. 1998; 36:212–215.33. Mattie H, Craig WA, Pechère JC. Determinants of efficacy and toxicity of aminoglycosides. J Antimicrob Chemother. 1989; 24:281–293.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Helicobacter pylori infection in functional dyspepsia
- Response to Treatment of Helicobacter pylori-associated Dyspepsia: Eradication of Helicobacter pylori or Correction of Gastric or Intestinal Dysbiosis?
- Treatment of Helicobacter pylori infection
- Helicobacter pylori Infection and Cardiovascular Disease
- Treatment of Helicobacter pylori infection