Ann Surg Treat Res.
2019 Apr;96(4):169-176. 10.4174/astr.2019.96.4.169.
Nomogram for accurate prediction of breast and axillary pathologic response after neoadjuvant chemotherapy in node positive patients with breast cancer
- Affiliations
-
- 1Department of Surgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 2Division of Breast Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sekyung.lee@samsung.com
- KMID: 2442211
- DOI: http://doi.org/10.4174/astr.2019.96.4.169
Abstract
- PURPOSE
Many patients with cytology proven node-positive breast cancer receive a neoadjuvant chemotherapy (NAC) treatment. We developed a nomogram to predict the breast and axillary pathologic complete responses (pCR) in patients with a cytologically proven axillary node positive breast cancer with NAC.
METHODS
We selected 995 patients who were diagnosed with an invasive breast cancer and axillary lymph nodes metastasis, and who were treated with NAC followed by a curative surgery at the Samsung Medical Center between January 2007 and December 2014. The baseline patient and tumor characteristics, chemotherapy regimen, and tumor and nodal responses were thoroughly analyzed and reviewed. A nomogram was developed using a binary logistic regression model with a cross validation.
RESULTS
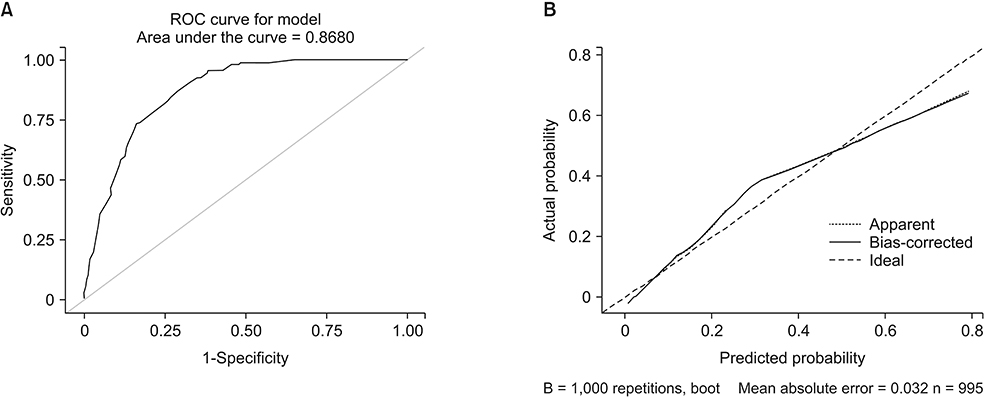
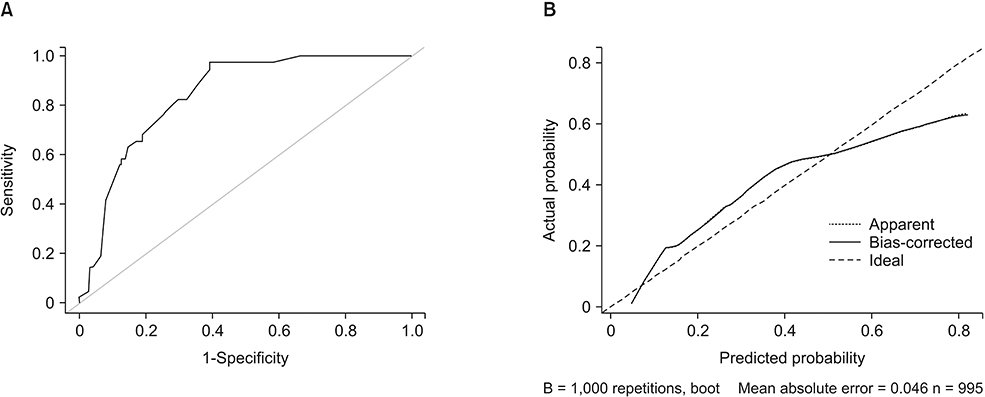
Axillary pCR was achieved in 47.3% and breast pCR was achieved in 24.3% of the patients after NAC. In this case, the both pCR was associated with an initial clinical tumor stage, negative progesterone receptor status, positive human epidermal growth factor receptor 2 status, and clinical radiologic nodal responses. A nomogram was developed based on the clinical and statistically significant predictors. It had good discrimination performance (area under the curve [AUC], 0.868; 95% confidence interval, 0.84-0.89) and calibration fit as noted in that case. The cross validation had an average AUC 0.853 (0.837-0.869).
CONCLUSION
Our nomogram might help to predict breast and axillary pCRs after NAC in patients with an initially node-positive breast cancer. Minimal surgery might be acceptable in patients for whom the nomogram indicates a high probability of achieving pCRs.
Keyword
MeSH Terms
-
Area Under Curve
Breast Neoplasms*
Breast*
Calibration
Discrimination (Psychology)
Drug Therapy*
Humans
Logistic Models
Lymph Nodes
Neoadjuvant Therapy
Neoplasm Metastasis
Nomograms*
Polymerase Chain Reaction
Receptor, Epidermal Growth Factor
Receptors, Progesterone
Receptor, Epidermal Growth Factor
Receptors, Progesterone
Figure
Cited by 1 articles
-
Targeted axillary biopsy and sentinel lymph node biopsy for axillary restaging after neoadjuvant chemotherapy
Gunay Gurleyik, Sibel Aydin Aksu, Fügen Aker, Kubra Kaytaz Tekyol, Eda Tanrikulu, Emin Gurleyik
Ann Surg Treat Res. 2021;100(6):305-312. doi: 10.4174/astr.2021.100.6.305.
Reference
-
1. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997; 15:2483–2493.
Article2. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006; 24:2019–2027.
Article3. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013; 310:1455–1461.4. Mittendorf EA, Caudle AS, Yang W, Krishnamurthy S, Shaitelman S, Chavez-MacGregor M, et al. Implementation of the american college of surgeons oncology group z1071 trial data in clinical practice: is there a way forward for sentinel lymph node dissection in clinically node-positive breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol. 2014; 21:2468–2473.
Article5. Kim KS, Kim Z, Shim EJ, Kim NH, Jung SY, Kim J, et al. The reality in the follow-up of breast cancer survivors: survey of Korean Breast Cancer Society. Ann Surg Treat Res. 2015; 88:133–139.
Article6. Shen J, Gilcrease MZ, Babiera GV, Ross MI, Meric-Bernstam F, Feig BW, et al. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer. 2007; 109:1255–1263.
Article7. Hennessy BT, Hortobagyi GN, Rouzier R, Kuerer H, Sneige N, Buzdar AU, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005; 23:9304–9311.
Article8. Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002; 20:1304–1310.
Article9. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006; 98:599–609.
Article10. Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007; 25:3657–3663.
Article11. Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005; 23:5983–5992.
Article12. Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014; 260:608–614.13. Broglio KR, Quintana M, Foster M, Olinger M, McGlothlin A, Berry SM, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016; 2:751–760.14. Vila J, Mittendorf EA, Farante G, Bassett RL, Veronesi P, Galimberti V, et al. Nomograms for predicting axillary response to neoadjuvant chemotherapy in clinically node-positive patients with breast cancer. Ann Surg Oncol. 2016; 23:3501–3509.
Article15. Kantor O, Sipsy LM, Yao K, James TA. A predictive model for axillary node pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2018; 25:1304–1311.
Article16. Kim JY, Park HS, Kim S, Ryu J, Park S, Kim SI. Prognostic nomogram for prediction of axillary pathologic complete response after neoadjuvant chemotherapy in cytologically proven node-positive breast cancer. Medicine (Baltimore). 2015; 94:e1720.
Article17. Schipper RJ, Moossdorff M, Nelemans PJ, Nieuwenhuijzen GA, de Vries B, Strobbe LJ, et al. A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo(immuno) therapy in patients with clinically node-positive breast cancer. Clin Breast Cancer. 2014; 14:315–322.18. Cortazar P, Geyer CE Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol. 2015; 22:1441–1446.
Article19. Donker M, Straver ME, Wesseling J, Loo CE, Schot M, Drukker CA, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015; 261:378–382.20. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016; 34:1072–1078.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Accuracy of Radiological Axillary Staging for Breast Cancer Patients with Neoadjuvant Chemotherapy
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update
- Neoadjuvant Chemotherapy Decreases the Identification Rate of Sentinel Lymph Node Biopsy
- Axillary sparganosis which was misunderstood lymph node metastasis during neoadjuvant chemotheraphy in a breast cancer patient